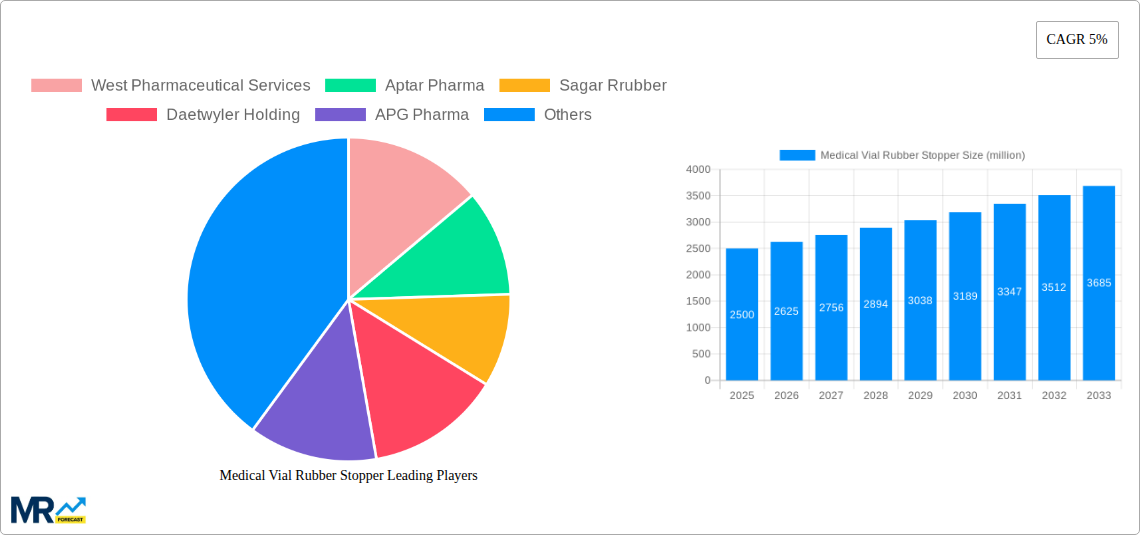
1. What is the projected Compound Annual Growth Rate (CAGR) of the Medical Vial Rubber Stopper?
The projected CAGR is approximately 5%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Medical Vial Rubber Stopper
Medical Vial Rubber StopperMedical Vial Rubber Stopper by Type (Butyl Rubber, EPDM, Natural Rubber, Others), by Application (Injection Vials, Infusion Vials, Freeze Dry Vials, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
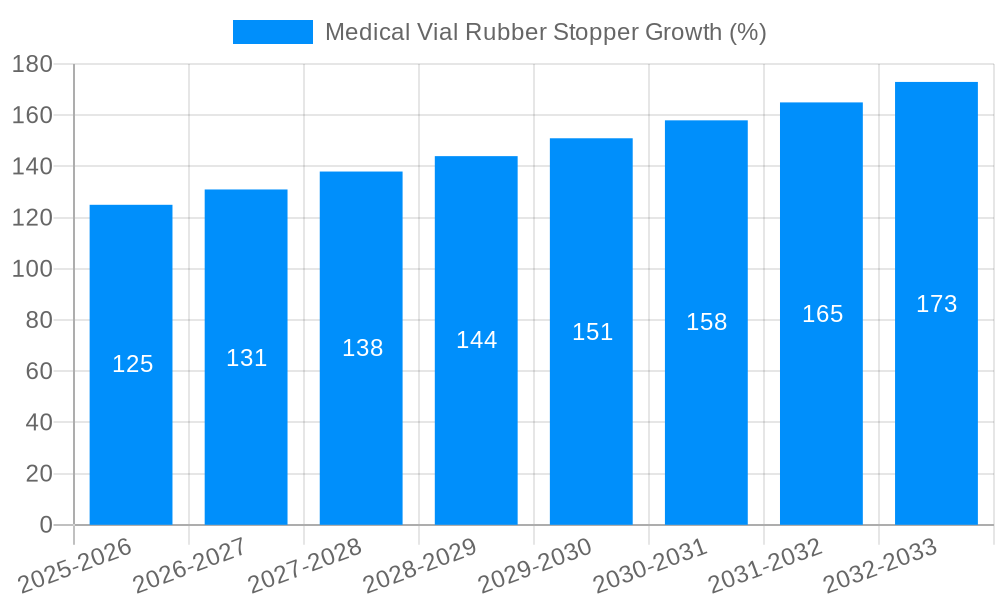
The global Medical Vial Rubber Stopper market is projected to experience significant growth, driven by the increasing demand for sterile and safe packaging solutions in the pharmaceutical and biopharmaceutical industries. With an estimated market size of USD 2.5 billion in 2025, the sector is poised for expansion, fueled by advancements in drug delivery systems and a rising prevalence of chronic diseases necessitating robust pharmaceutical packaging. The market is anticipated to grow at a Compound Annual Growth Rate (CAGR) of 5% from 2025 to 2033, reaching an estimated value of USD 3.7 billion by 2033. Key drivers include the escalating production of vaccines, biologics, and injectable drugs, which rely heavily on high-quality rubber stoppers for vial integrity and product safety. Furthermore, stringent regulatory requirements for pharmaceutical packaging, emphasizing sterility, biocompatibility, and chemical inertness, are propelling the adoption of advanced stopper materials and manufacturing processes. The increasing focus on personalized medicine and the development of novel therapeutic agents further underscore the importance of reliable vial closures, contributing to sustained market momentum.
The market is segmented by material type and application, reflecting diverse industry needs. Butyl rubber and EPDM dominate the material segment due to their excellent chemical resistance, low permeability, and high elasticity, making them ideal for a wide range of pharmaceutical applications including injection vials, infusion vials, and freeze-dry vials. The growing preference for lyophilized drugs, particularly in the biopharmaceutical sector, is a significant trend bolstering the demand for specialized stoppers suited for freeze-drying processes. While the market benefits from strong growth drivers, it faces certain restraints. Fluctuations in raw material prices, particularly for natural and synthetic rubbers, can impact manufacturing costs. Additionally, the development of alternative sterile packaging solutions and the rigorous qualification processes for new stopper materials can pose challenges to rapid market penetration. However, the continuous innovation in stopper technology, including the development of low-extractable and drug-compatible stoppers, is expected to mitigate these restraints and ensure a positive growth trajectory for the medical vial rubber stopper market.
This report provides an in-depth analysis of the global Medical Vial Rubber Stopper market, examining trends, drivers, challenges, and future prospects. Spanning a study period from 2019 to 2033, with a base year of 2025 and a forecast period from 2025 to 2033, this research leverages extensive historical data from 2019-2024 to deliver actionable insights. The market is projected to witness significant growth, with estimated revenues reaching into the tens of millions of USD by the end of the forecast period, driven by increasing pharmaceutical production and advancements in drug delivery systems.
The global Medical Vial Rubber Stopper market is experiencing a dynamic evolution, shaped by a confluence of technological advancements, stringent regulatory landscapes, and the ever-growing demand for safe and reliable pharmaceutical packaging solutions. A key trend observed is the increasing preference for advanced elastomeric materials, particularly butyl rubber, due to its superior barrier properties, low permeability to gases and moisture, and excellent compatibility with a wide range of pharmaceutical formulations. This has led to its dominant position across various applications, including injection vials and freeze-dry vials. The market is also witnessing a surge in the demand for stoppers designed for specific drug delivery methods, such as lyophilized drug products, which require stoppers with enhanced stability and minimal particle shedding during the freeze-drying process. Furthermore, the growing emphasis on patient safety and drug integrity is driving the adoption of innovative stopper designs that offer improved sealing capabilities, reduced extractables and leachables, and features that prevent contamination. The pharmaceutical industry's relentless pursuit of cost-effectiveness and operational efficiency is also influencing trends, leading to the development of stoppers that can withstand advanced sterilization techniques and automated filling processes. Sustainability is emerging as another significant trend, with manufacturers exploring eco-friendly materials and production methods for rubber stoppers, albeit at a nascent stage of adoption, as the primary focus remains on pharmaceutical-grade quality and regulatory compliance. The market is expected to witness a steady upward trajectory, with the Injection Vials segment continuing to be the largest application due to the widespread use of injectable drugs across diverse therapeutic areas. Innovations in stopper coatings and surface treatments to further enhance inertness and reduce interaction with sensitive drug formulations are also gaining traction.
The market's trajectory is significantly influenced by the increasing complexity of drug formulations, including biologics and personalized medicines, which necessitate specialized packaging solutions. This has spurred innovation in stopper design and material science. The global healthcare expenditure growth, coupled with an aging population and the rising prevalence of chronic diseases, is directly translating into higher demand for pharmaceutical products, thereby boosting the need for essential packaging components like rubber stoppers. Moreover, advancements in drug manufacturing technologies, such as continuous manufacturing, are demanding packaging components that can seamlessly integrate into high-speed, automated production lines, further driving innovation in stopper design for optimal performance and compatibility. The increasing focus on supply chain security and the need to prevent counterfeiting are also indirectly contributing to the demand for high-quality, tamper-evident stopper solutions. As the pharmaceutical industry continues to expand its global reach, particularly in emerging economies, the demand for reliable and cost-effective rubber stoppers is poised for substantial growth.
The medical vial rubber stopper market is experiencing robust growth, propelled by a multifaceted interplay of factors that underscore the critical role of these components in modern pharmaceutical packaging. A primary driving force is the unprecedented expansion of the global pharmaceutical industry, fueled by increasing healthcare expenditure, an aging global population, and the rising incidence of chronic diseases. This burgeoning pharmaceutical production directly translates into a higher demand for vials and, consequently, the rubber stoppers that ensure their integrity and sterility. The development and commercialization of new drugs, particularly biologics, vaccines, and advanced therapeutic agents, which often require specialized sterile packaging, further amplify this demand. The growing emphasis on biosimilars and generic drug production also contributes significantly, as these sectors aim to provide affordable alternatives, thus increasing the overall volume of pharmaceutical products requiring reliable containment. Furthermore, the increasing adoption of pre-filled syringes and advanced drug delivery systems necessitates high-quality stoppers that can maintain product stability and prevent leakage. Regulatory mandates and the unwavering commitment to patient safety and drug efficacy are also crucial drivers. Healthcare authorities worldwide enforce stringent quality standards for pharmaceutical packaging, pushing manufacturers to invest in advanced materials and production processes that guarantee inertness, low extractables, and superior sealing properties. The need to ensure drug integrity throughout the supply chain, from manufacturing to patient administration, underpins the demand for premium rubber stoppers that can withstand various storage conditions and handling processes.
The continued investment in research and development by pharmaceutical companies to bring innovative therapies to market acts as a constant catalyst. Each new drug formulation, especially those requiring precise dosing and sterile administration, translates into a requirement for meticulously designed and manufactured stoppers. The expansion of healthcare infrastructure in emerging economies is also a significant factor, as increased access to medicines drives up the overall consumption of pharmaceutical products. This geographical expansion of pharmaceutical manufacturing and consumption is creating new markets and opportunities for medical vial rubber stopper suppliers. The growing preference for parenteral drug administration, due to its rapid onset of action and high bioavailability, is another key contributor. This trend necessitates a reliable and secure sealing mechanism, which is precisely what high-quality rubber stoppers provide. The continuous innovation in materials science and manufacturing technologies for rubber stoppers, aimed at improving their performance, reducing particulate generation, and enhancing compatibility with diverse drug formulations, further fuels market growth by offering enhanced solutions to the pharmaceutical industry's evolving needs.
Despite the promising growth trajectory, the medical vial rubber stopper market is not without its hurdles. A significant challenge lies in the stringent and evolving regulatory landscape governing pharmaceutical packaging materials. Manufacturers must constantly adhere to evolving Good Manufacturing Practices (GMPs) and meet the rigorous requirements of regulatory bodies like the FDA, EMA, and others. This necessitates significant investment in quality control, validation, and compliance, which can increase production costs and time-to-market for new products. The high cost of raw materials, particularly high-grade butyl rubber, and the volatility in their pricing can impact profit margins and create supply chain uncertainties. Fluctuations in crude oil prices, which are linked to the production of synthetic rubbers, can directly affect the cost of these essential materials. Furthermore, the growing concern over extractables and leachables (E&L) from rubber stoppers is a major restraint. Pharmaceutical companies are increasingly scrutinizing packaging components for potential contamination of drug products, leading to a demand for stoppers with minimal E&L profiles. Developing and validating stoppers that meet these ultra-low E&L requirements can be complex and expensive, requiring specialized testing and advanced material formulations. The intense competition within the market, characterized by the presence of numerous global and regional players, can lead to price pressures and hinder profitability, especially for smaller manufacturers. The need for specialized manufacturing capabilities and infrastructure further creates a barrier to entry for new players.
The development of innovative stopper designs, while a growth driver, also presents challenges. The transition from traditional stopper designs to more advanced, specialized ones requires significant investment in R&D, tooling, and process optimization. Ensuring the compatibility of new stopper materials and designs with a wide array of sensitive drug formulations, including biologics and cell therapies, requires extensive testing and validation, which can be a time-consuming and costly endeavor. Moreover, the global supply chain disruptions, as witnessed in recent years, can impact the availability of raw materials and finished products, leading to production delays and increased lead times. The requirement for specialized sterilization processes for rubber stoppers, such as autoclaving or gamma irradiation, also adds to the complexity and cost of production. The increasing demand for tamper-evident features and child-resistant packaging, while beneficial for product security, also introduces design and manufacturing complexities that can increase costs. Finally, the pressure to adopt sustainable manufacturing practices, while a positive long-term trend, can also be a challenge in the short to medium term, as it may require significant upfront investment in new technologies and processes, particularly for established manufacturers.
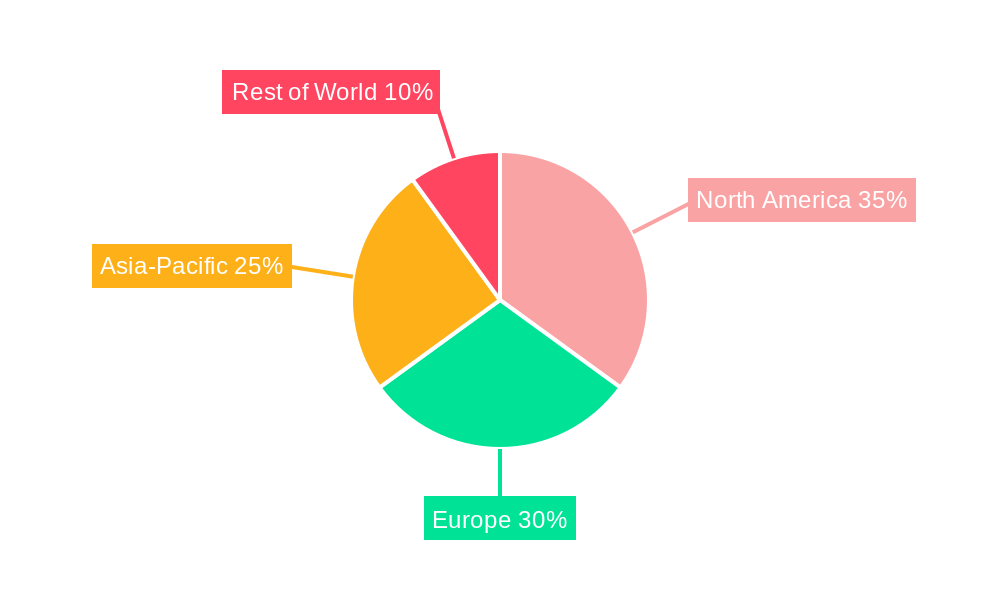
The global Medical Vial Rubber Stopper market is characterized by the dominance of specific regions and product segments, driven by a combination of pharmaceutical manufacturing prowess, healthcare infrastructure development, and regulatory alignment.
Key Dominating Segment:
Key Dominating Regions:
North America (United States and Canada): This region consistently holds a significant share of the medical vial rubber stopper market.
Europe: Another leading region, Europe exhibits strong demand driven by its significant pharmaceutical manufacturing base and developed healthcare systems.
The dominance of these regions and the Injection Vials segment underscores the critical role of robust pharmaceutical manufacturing capabilities, advanced healthcare infrastructure, and stringent quality compliance in shaping the global medical vial rubber stopper market.
The medical vial rubber stopper industry is propelled by several key growth catalysts. The expanding global pharmaceutical market, driven by an aging population, rising chronic disease prevalence, and increased healthcare spending, directly translates to higher demand for injectable drugs and thus, their stoppers. The continuous development and launch of new drugs, especially biologics and vaccines, which often require specialized sterile packaging, act as significant catalysts. Advancements in drug delivery systems, such as pre-filled syringes and lyophilized formulations, necessitate the use of high-performance, reliable stoppers. Furthermore, stringent regulatory requirements emphasizing patient safety and drug integrity compel pharmaceutical manufacturers to opt for premium, validated rubber stoppers, fostering innovation and market growth.
This report offers a comprehensive examination of the medical vial rubber stopper market, providing granular insights into its multifaceted dynamics. It delves into the intricate details of market segmentation by type, application, and region, offering detailed analyses of the historical performance (2019-2024), current landscape (2025), and future projections (2025-2033). The report elucidates the key trends shaping the market, such as the increasing preference for advanced elastomeric materials and specialized stopper designs for complex drug formulations. It also thoroughly investigates the driving forces, including the expansion of the global pharmaceutical industry and the continuous pipeline of new drug development, alongside the challenges and restraints like stringent regulations and raw material cost volatility. Furthermore, the report highlights the dominant regions and segments, identifies key market players, and meticulously details significant industry developments, providing a holistic understanding of this critical sector within pharmaceutical packaging.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 5%.
Key companies in the market include West Pharmaceutical Services, Aptar Pharma, Sagar Rrubber, Daetwyler Holding, APG Pharma, Daikyo Seiko, Bormioli Pharma, Adelphi Healthcare Packaging, Origin Pharma Packaging, Shandong Pharmaceutical Glass, Jiangsu Hualan New Pharmaceutical Material, Hebei First Rubber Medical Technology, Anhui Huafeng Pharmaceutical Rubber Co., Ltd, .
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Medical Vial Rubber Stopper," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Medical Vial Rubber Stopper, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.