1. What is the projected Compound Annual Growth Rate (CAGR) of the Yellow Fever Vaccines?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Yellow Fever Vaccines
Yellow Fever VaccinesYellow Fever Vaccines by Application (Routine Immunization, Travelers, Other), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
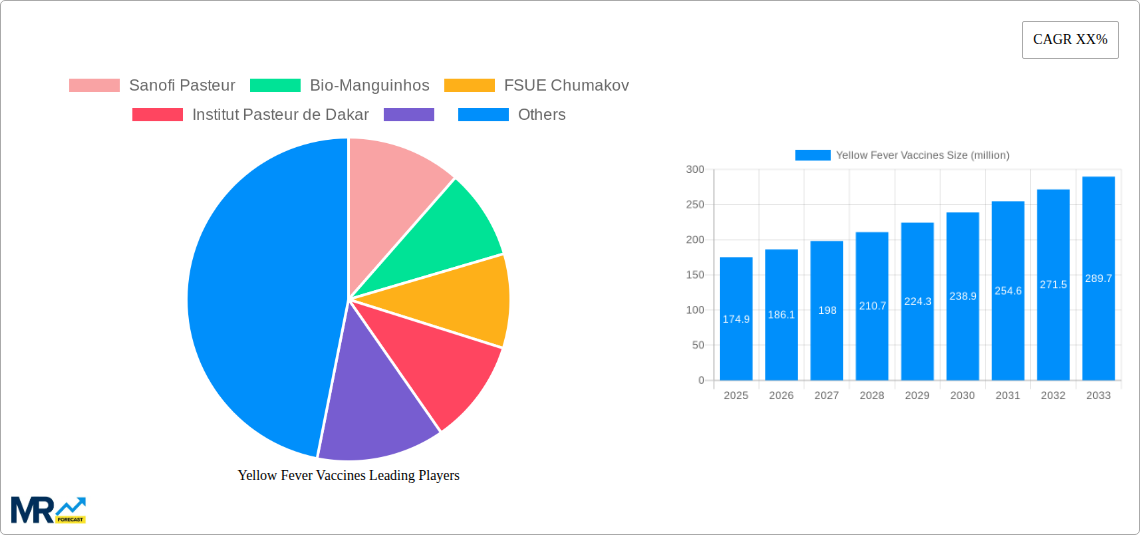
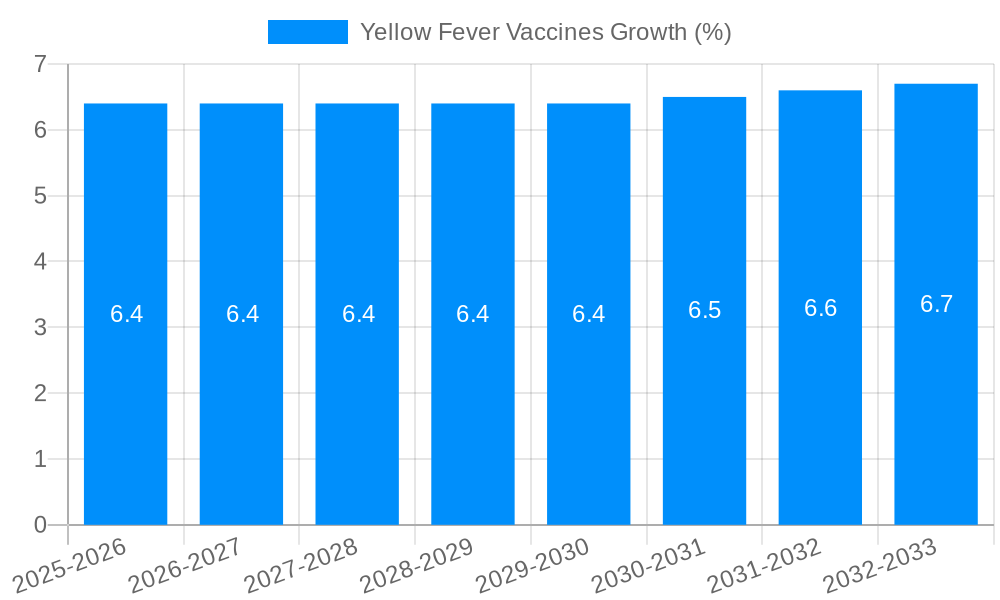
The global Yellow Fever Vaccines market is poised for substantial growth, estimated at a current market size of $174.9 million and projected to expand at a Compound Annual Growth Rate (CAGR) of approximately 6.5% over the forecast period of 2025-2033. This robust expansion is driven by a confluence of factors including increasing global travel, the persistent threat of endemic yellow fever in tropical regions, and enhanced vaccination campaigns orchestrated by governments and international health organizations. Routine immunization programs, particularly in countries with endemic or at-risk populations, form the backbone of market demand. Furthermore, a growing segment of international travelers, motivated by both leisure and business, seek preventative measures against yellow fever, thereby contributing significantly to market dynamics. The strategic initiatives by key players such as Sanofi Pasteur and Bio-Manguinhos to expand manufacturing capacities and distribution networks are instrumental in meeting the rising global demand and ensuring accessibility of these life-saving vaccines.
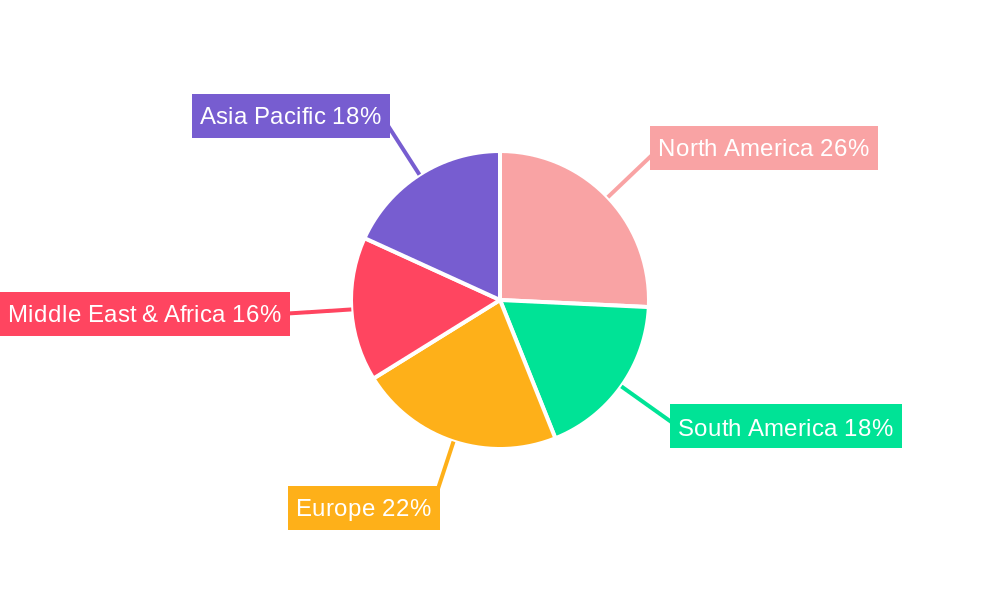
The market's trajectory is further shaped by evolving public health policies and a heightened awareness of vaccine preventable diseases. While the market benefits from strong drivers, certain restraints such as the potential for vaccine hesitancy in some regions and the stringent regulatory approval processes can present challenges. However, the inherent efficacy of yellow fever vaccines in preventing a severe and potentially fatal disease, coupled with the WHO's recommendations for vaccination in endemic and outbreak-prone areas, underpins the market's resilience. The market is segmented by application, with routine immunization holding the dominant share, followed by traveler vaccinations. Geographically, Asia Pacific, with its large population and increasing international connectivity, is expected to witness significant growth, alongside established markets in North America and Europe, driven by robust healthcare infrastructure and ongoing vaccination efforts.
The global yellow fever vaccine market is poised for significant expansion and evolution over the study period of 2019-2033, with a projected surge from its base year of 2025. XXX highlights a burgeoning demand driven by increasing global travel, heightened awareness of the disease's potential for epidemic outbreaks, and strengthened public health initiatives in endemic and at-risk regions. The market is characterized by a growing preference for highly effective and safe vaccines, alongside a sustained emphasis on accessibility and affordability, particularly in low-resource settings. During the historical period of 2019-2024, the market experienced steady growth, underpinned by routine immunization programs in affected African and South American nations. The estimated year of 2025 is anticipated to witness a substantial uptick, setting a strong trajectory for the forecast period of 2025-2033. Innovation in vaccine technology, including the exploration of novel delivery methods and extended duration of immunity, is a key trend shaping the future landscape. Furthermore, the increasing global interconnectedness and the potential for international spread of yellow fever underscore the persistent need for robust vaccination strategies. The market is also observing strategic collaborations between manufacturers and public health organizations to ensure adequate supply and efficient distribution, especially during outbreak scenarios. The regulatory landscape, while generally supportive of vaccine development, also plays a crucial role in ensuring product quality and safety, which in turn influences market dynamics. Emerging economies, as they continue to develop their healthcare infrastructure and prioritize preventative health measures, are expected to contribute significantly to market growth. The overall trend indicates a market that is not only growing in volume but also maturing in its approach, with a focus on sustainable supply chains and long-term disease prevention. The successful eradication or containment of yellow fever outbreaks relies heavily on the availability and uptake of these vaccines, making it a critical public health tool.
The yellow fever vaccine market is experiencing a potent confluence of driving forces that are collectively propelling its growth. A primary catalyst is the increasing incidence and geographical spread of mosquito-borne diseases, including yellow fever, often exacerbated by climate change, urbanization, and human encroachment into vector habitats. This heightened risk necessitates proactive vaccination campaigns and a robust supply of vaccines. Secondly, growing global travel and international commerce are significant drivers. As more individuals travel to and from endemic regions, the risk of importing and spreading the disease across borders increases, prompting advisory bodies and governments to strongly recommend or mandate yellow fever vaccination for travelers, thereby boosting demand for the "Travelers" segment. Furthermore, strengthened global and national public health initiatives aimed at disease prevention and control are paramount. Organizations like the WHO are actively promoting yellow fever vaccination as part of routine immunization schedules in at-risk countries and are often involved in outbreak response efforts that require rapid vaccine deployment. The expanding healthcare infrastructure and increasing disposable incomes in emerging economies are also contributing, as these regions are better equipped to invest in public health programs and individuals have greater access to healthcare services, including vaccinations. Finally, ongoing research and development efforts leading to improved vaccine formulations, longer-lasting immunity, and potentially more cost-effective production methods are further stimulating market interest and adoption.
Despite the promising growth trajectory, the yellow fever vaccine market faces several significant challenges and restraints that could temper its expansion. A primary concern is the limited number of manufacturers capable of producing WHO-prequalified yellow fever vaccines. This concentration of production can lead to supply chain vulnerabilities and potential shortages, especially during periods of heightened demand or unexpected outbreaks, impacting the ability to meet the market's needs. Another critical restraint is the potential for adverse events and contraindications associated with live-attenuated vaccines, such as the current dominant yellow fever vaccine (YF-17D). While rare, severe neurological or viscerotropic adverse events can lead to public hesitancy and a reluctance to vaccinate in some populations, necessitating rigorous post-market surveillance and clear communication strategies. Geopolitical instability and funding challenges in some endemic regions can also hinder the sustained implementation of vaccination programs. Countries facing economic hardship or political turmoil may struggle to allocate sufficient resources for vaccine procurement, distribution, and the necessary infrastructure to support widespread immunization. Furthermore, the high cost of vaccine development and the stringent regulatory approval processes can deter new entrants into the market, thereby limiting competition and potentially affecting pricing. Lastly, inadequate cold chain infrastructure and logistical challenges in remote or resource-limited areas can impede the effective delivery and storage of vaccines, leading to wastage and reduced vaccine efficacy, thereby posing a significant hurdle to achieving herd immunity.
The Routine Immunization segment, particularly within the African continent, is poised to be a dominant force in the global yellow fever vaccine market. This dominance stems from a multifaceted interplay of endemicity, historical vaccination efforts, and ongoing public health priorities.
Africa bears the brunt of the yellow fever disease burden, with numerous countries designated as high-risk or endemic. The established presence of yellow fever virus in various regions necessitates continuous, robust vaccination programs to prevent devastating outbreaks and maintain disease control. The World Health Organization (WHO) and other international health organizations have long prioritized yellow fever prevention in Africa, leading to the integration of the vaccine into national immunization schedules. This long-standing commitment translates into a sustained and significant demand for yellow fever vaccines.
The Routine Immunization segment is intrinsically linked to this African dominance. These programs are designed for long-term disease prevention, targeting infants, children, and adolescents to build population-level immunity. Unlike ad-hoc outbreak responses or traveler-specific vaccinations, routine immunization represents a consistent and predictable demand for vaccines. Countries with established Expanded Programs on Immunization (EPIs) already possess the infrastructure for vaccine delivery, including healthcare worker training, cold chain maintenance, and distribution networks, which facilitates the ongoing administration of yellow fever vaccines.
Furthermore, the sheer population size of many African nations contributes to the scale of the routine immunization segment. Even with relatively high vaccination coverage, the large number of individuals requiring primary vaccination or booster doses (where applicable) translates into substantial vaccine volumes.
Specific countries within Africa that are likely to exhibit particularly strong demand within this segment include:
Beyond Africa, other regions like South America, particularly countries with endemic yellow fever transmission such as Brazil and Peru, also contribute significantly to the Routine Immunization segment. However, the historical and ongoing burden of the disease, coupled with the scale of public health interventions, positions Africa as the primary driver for this segment.
The synergy between the Routine Immunization segment and the African continent is expected to remain the cornerstone of the yellow fever vaccine market throughout the forecast period. This consistent, large-scale demand underpins the market's overall growth and stability, influencing production volumes, supply chain strategies, and the focus of public health interventions.
The growth of the yellow fever vaccine industry is significantly propelled by key catalysts. A primary driver is the increasing recognition of yellow fever's pandemic potential, amplified by global travel patterns and climate change, which encourages proactive vaccination strategies. Furthermore, strengthened global health initiatives and partnerships, such as those led by the WHO, are instrumental in promoting vaccination and ensuring vaccine availability in endemic regions. Investments in improved vaccine technologies and production capabilities, aimed at enhancing efficacy and affordability, also fuel market expansion. Finally, the growing emphasis on preventative healthcare and disease surveillance in at-risk countries creates a sustained demand for yellow fever vaccines.
This report provides an in-depth analysis of the global yellow fever vaccine market, offering comprehensive coverage of its multifaceted landscape. It delves into the historical performance from 2019-2024, establishing a baseline for the market's trajectory, and forecasts future trends and opportunities from 2025-2033, with 2025 serving as the base and estimated year. The report meticulously examines the driving forces, challenges, and restraints shaping the market's dynamics, offering critical insights into the factors influencing demand and supply. A detailed exploration of key regional and segmental dominance, with a particular focus on the "Routine Immunization" segment, highlights areas of significant market potential and strategic importance. Furthermore, the report identifies crucial growth catalysts and profiles the leading players and their contributions to the industry. Significant developments and past trends are also cataloged, providing a holistic understanding of the market's evolution. This comprehensive overview is designed to equip stakeholders with the knowledge necessary for informed decision-making and strategic planning in the vital yellow fever vaccine sector.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Sanofi Pasteur, Bio-Manguinhos, FSUE Chumakov, Institut Pasteur de Dakar, .
The market segments include Application.
The market size is estimated to be USD 174.9 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Yellow Fever Vaccines," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Yellow Fever Vaccines, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.