1. What is the projected Compound Annual Growth Rate (CAGR) of the Epinephrine for Anaphylaxis Treatment Market?
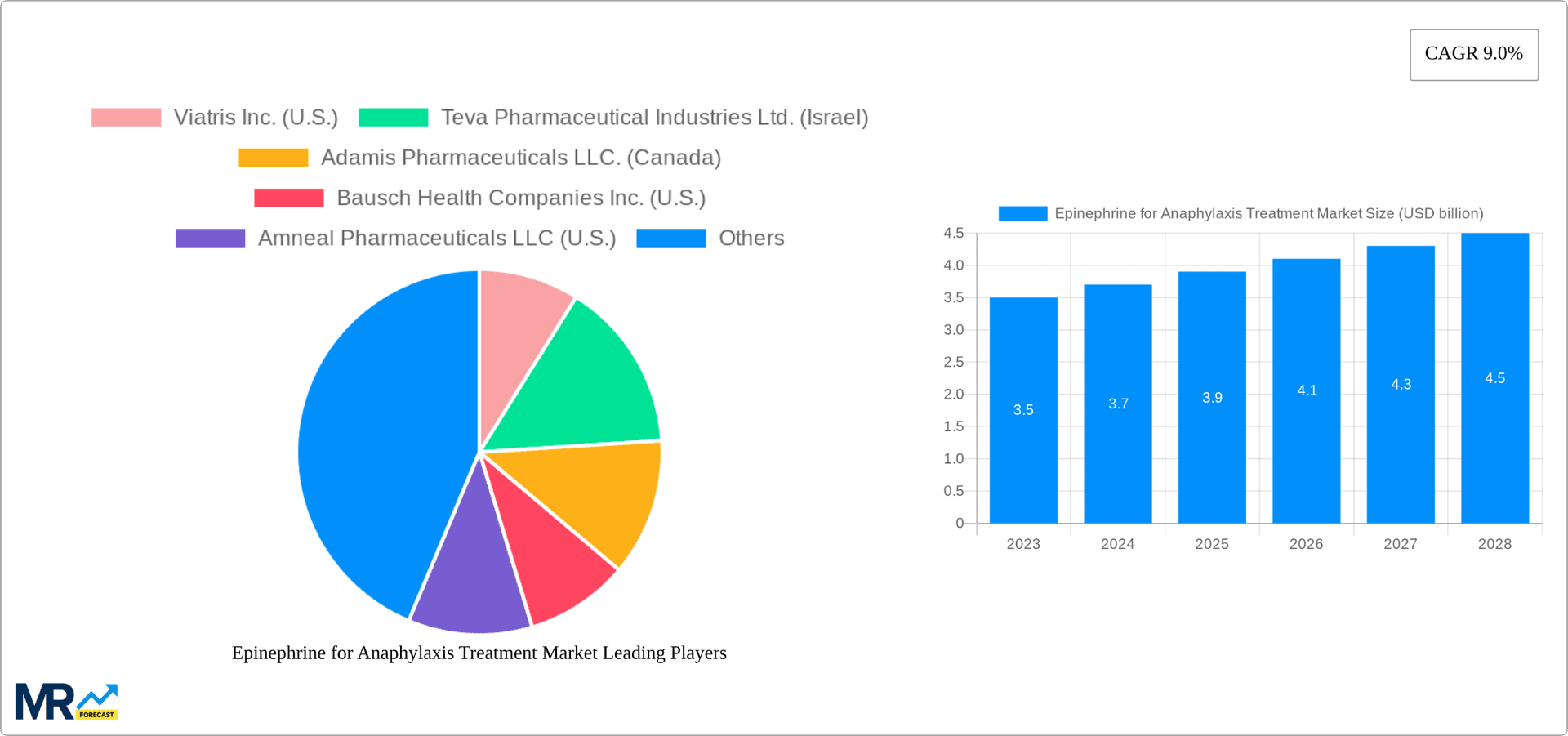
The projected CAGR is approximately 9.0%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Epinephrine for Anaphylaxis Treatment Market
Epinephrine for Anaphylaxis Treatment MarketEpinephrine for Anaphylaxis Treatment Market by Product Type (Autoinjectors, Prefilled Syringes, Others), by Type (Branded, Generics), by Distribution Channel (Hospital Pharmacies, Online & Retail Pharmacies), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
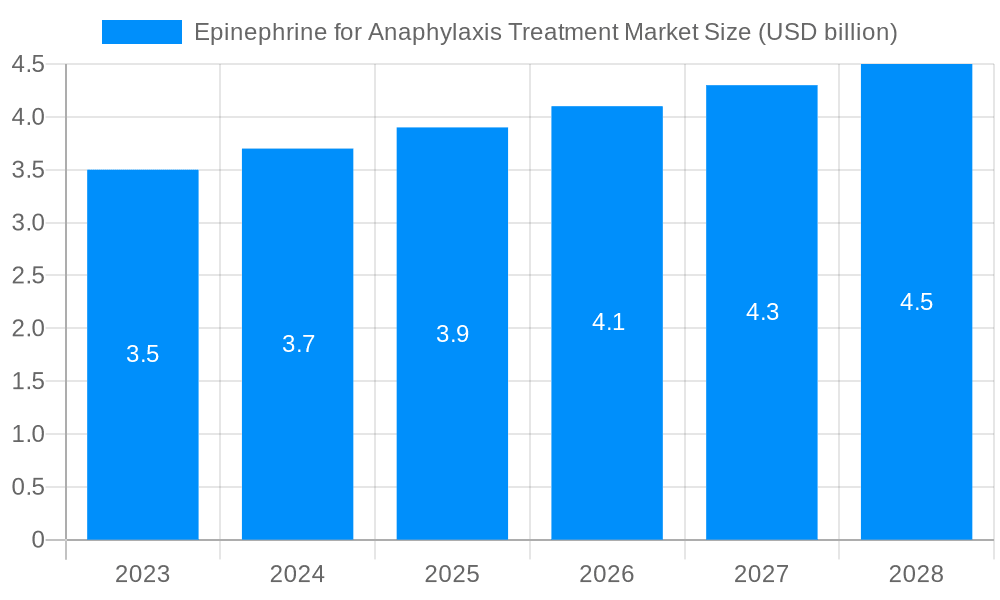
The size of the Epinephrine for Anaphylaxis Treatment Market was valued at USD 1.56 USD billion in 2023 and is projected to reach USD 2.85 USD billion by 2032, with an expected CAGR of 9.0% during the forecast period, reaching a value of 1.56 USD billion. The market's expansion is influenced by factors such as the increasing prevalence of anaphylaxis, growing awareness about the condition and its treatment, and the introduction of novel epinephrine auto-injectors. Moreover, government initiatives aimed at improving healthcare infrastructure, particularly in emerging economies, further contribute to market growth.

The epinephrine for anaphylaxis treatment market is characterized by dynamic shifts driven by strategic collaborations between pharmaceutical manufacturers and healthcare systems. These alliances are instrumental in broadening patient access to critical epinephrine auto-injectors, ensuring timely availability in emergency situations. Concurrently, continuous innovation in auto-injector technology, focusing on enhanced user-friendliness and more effective delivery mechanisms, is significantly improving the administration experience and overall efficacy of epinephrine. These advancements are poised to be key accelerators of market expansion.

A primary catalyst for the epinephrine for anaphylaxis treatment market is the alarming rise in anaphylactic reactions, largely attributed to an increasing incidence of food allergies and severe reactions to insect stings. Augmented public awareness, bolstered by extensive awareness campaigns and educational outreach, is fostering a greater understanding of anaphylaxis and the critical role of readily accessible epinephrine auto-injectors. This heightened awareness directly translates into a more robust demand for these life-saving devices.
Moreover, proactive government initiatives and supportive healthcare policies, designed to bolster emergency preparedness and mitigate anaphylaxis-related mortality rates, are significantly contributing to market expansion. Governmental bodies globally are actively implementing regulations to guarantee the availability of epinephrine auto-injectors in pivotal public spaces, including educational institutions, workplaces, and community hubs, thereby further stimulating market growth.
A notable impediment to the epinephrine for anaphylaxis treatment market is the substantial cost associated with epinephrine auto-injectors, which can present a significant financial burden for individuals requiring consistent access to these essential devices. Additionally, concerns surrounding the potential for off-label use or inappropriate administration of epinephrine, particularly in self-administered scenarios, could act as a constraint on market expansion.
The North American region is expected to maintain its leadership position in the global epinephrine for anaphylaxis treatment market. This dominance is underpinned by a high prevalence of anaphylaxis cases and the presence of a well-developed and advanced healthcare infrastructure. Within North America, the United States plays a crucial role, driven by a high incidence of food allergies and the widespread adoption of sophisticated medical technologies.
Regarding market segmentation, the auto-injector product category is projected to lead the market throughout the forecast period. Auto-injectors are highly valued for their convenience and ease of use in emergency settings, making them the preferred choice for both patients and healthcare professionals. Branded epinephrine products are anticipated to command a larger market share than generic alternatives, often due to perceptions of superior efficacy and safety associated with established brands.
Research and development efforts focused on developing innovative epinephrine delivery systems and formulations are expected to drive market growth in the coming years. Additionally, the expansion of the market into emerging economies, where the incidence of anaphylaxis is on the rise, presents significant growth opportunities for market players.
Recent developments in the epinephrine for anaphylaxis treatment sector include:
This comprehensive report offers an in-depth analysis of the Epinephrine for Anaphylaxis Treatment Market, encompassing pivotal market trends, the primary driving forces behind its growth, prevalent challenges, detailed market segmentation, a thorough examination of the competitive landscape, and significant industry developments. The report furnishes invaluable insights for pharmaceutical companies, healthcare providers, and other stakeholders aiming to deepen their understanding of market dynamics and pinpoint emerging growth opportunities.
The data contained in this report is derived from a combination of primary and secondary sources. Primary research includes interviews with industry experts, market participants, and end-users. Secondary research involves the analysis of published data, company reports, government statistics, and other relevant sources.
The report provides a comprehensive analysis of the pricing dynamics of the Epinephrine for Anaphylaxis Treatment Market. It includes an assessment of the factors influencing pricing, such as raw material costs, manufacturing expenses, and distribution channels. The report also provides insights into the pricing strategies adopted by key players and their impact on the market.
This report offers an analysis of the import and export trends of the Epinephrine for Anaphylaxis Treatment Market. It provides data on the major importing and exporting countries, as well as the factors influencing the trade dynamics of the market. The report also assesses the impact of import and export regulations on market growth.
The report provides a thorough examination of the patent and trademark landscape of the Epinephrine for Anaphylaxis Treatment Market. It identifies the key patents and trademarks held by market players and analyzes their impact on the competitive landscape. The report also provides insights into the strategies adopted by companies to protect their intellectual property rights.
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 9.0% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 9.0%.
Key companies in the market include Viatris Inc. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), Adamis Pharmaceuticals LLC. (Canada), Bausch Health Companies Inc. (U.S.), Amneal Pharmaceuticals LLC (U.S.), DMK Pharmaceuticals (U.S.), ALK-Abelló A/S (Denmark), BIOPROJET (France).
The market segments include Product Type, Type, Distribution Channel.
The market size is estimated to be USD 1.56 USD billion as of 2022.
Growing Prevalence of Anaphylaxis among General Population to Drive Demand for Epinephrine.
Growing Prevalence of Anaphylaxis among General Population to Drive Demand for Epinephrine.
Growing Prevalence of Anaphylaxis among General Population to Drive Demand for Epinephrine.
May 2023: DMK Pharmaceuticals announced its merger with DMK Pharmaceuticals Corporation. The merger aimed to commercialize products and the development of new drug candidates.
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4850, USD 5850, and USD 6850 respectively.
The market size is provided in terms of value, measured in USD billion and volume, measured in Units.
Yes, the market keyword associated with the report is "Epinephrine for Anaphylaxis Treatment Market," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Epinephrine for Anaphylaxis Treatment Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.