1. What is the projected Compound Annual Growth Rate (CAGR) of the Veterinary Biological Vaccines?
The projected CAGR is approximately 5.8%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Veterinary Biological Vaccines
Veterinary Biological VaccinesVeterinary Biological Vaccines by Application (Livestock, Swine, Chicken, Others), by Type (Live Attenuated Vaccines, Inactivated Vaccines, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The global veterinary biological vaccines market, currently valued at approximately $8.252 billion (2025), is projected to experience robust growth, exhibiting a Compound Annual Growth Rate (CAGR) of 5.8% from 2025 to 2033. This expansion is fueled by several key drivers. Increasing pet ownership globally, coupled with rising awareness regarding animal health and preventative care, significantly contributes to market growth. Furthermore, the growing prevalence of zoonotic diseases—diseases transmissible from animals to humans—is driving demand for effective vaccines to protect both animal and human populations. Advancements in vaccine technology, leading to more effective, safer, and easier-to-administer vaccines, are also boosting market penetration. The market is segmented by various vaccine types (e.g., viral, bacterial, combination), animal species (e.g., poultry, cattle, swine, companion animals), and geographical regions. Major players such as Merck, Zoetis, Boehringer Ingelheim, and Elanco are driving innovation and competition, while smaller companies contribute to regional market diversity. Regulatory approvals and stringent quality standards play a significant role in shaping market dynamics. The increasing focus on sustainable and environmentally friendly vaccine production methods will also influence future market trends.
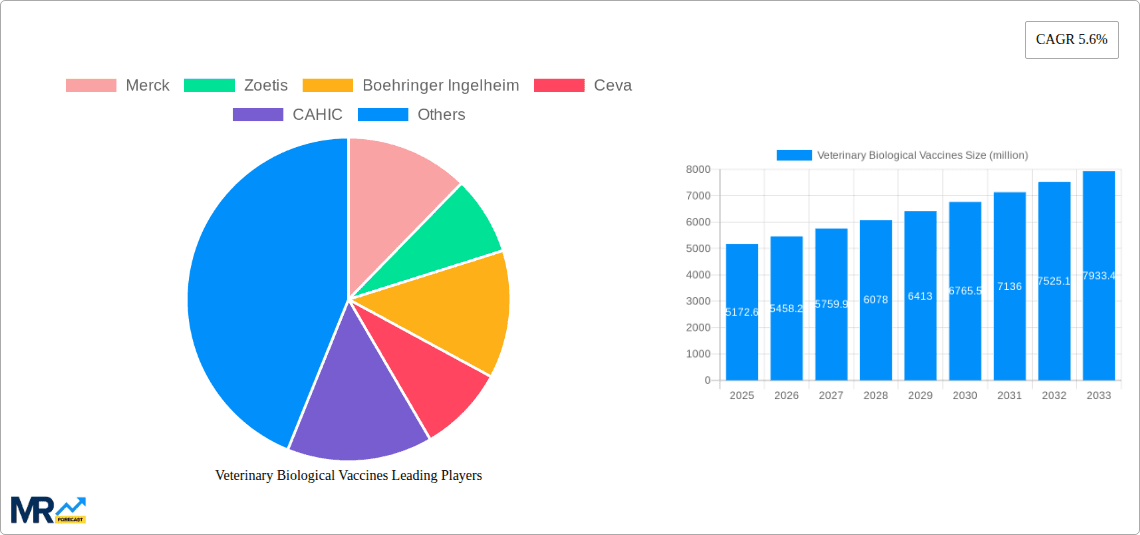

The competitive landscape is characterized by a mix of multinational corporations and smaller regional players. Established companies leverage their extensive research and development capabilities and global distribution networks to maintain market leadership. However, smaller companies, particularly in rapidly developing economies, are gaining traction through focusing on localized needs and cost-effective solutions. Challenges include maintaining a stable supply chain, addressing vaccine hesitancy among some pet owners and farmers, and navigating complex regulatory environments in different countries. Future market growth will be influenced by factors such as government initiatives promoting animal health, advancements in personalized medicine for animals, and the ongoing need for disease surveillance and prevention strategies. The market is expected to continue its upward trajectory, driven by the aforementioned factors and supported by a strong foundation of investment in research and development within the veterinary pharmaceutical industry.
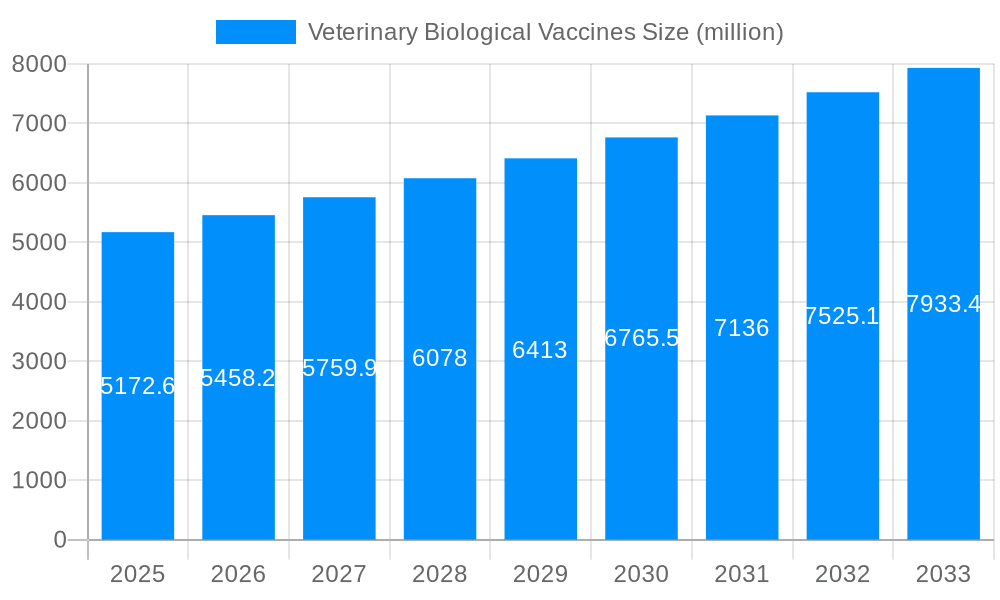

The global veterinary biological vaccines market is experiencing robust growth, projected to reach multi-billion dollar valuations by 2033. Driven by increasing pet ownership, heightened awareness of animal health, and expanding veterinary services, the market shows consistent expansion across various segments. The historical period (2019-2024) witnessed a steady rise in vaccine demand, particularly in developed regions with established pet healthcare infrastructure. The estimated year 2025 signals a significant market size in the millions, with forecasts (2025-2033) predicting continued substantial growth. This expansion is fueled not only by traditional vaccines against prevalent diseases like rabies and canine distemper but also by the development of novel vaccines targeting emerging infectious diseases and tailored to specific animal species. Furthermore, the increasing adoption of advanced technologies in vaccine development and manufacturing, such as recombinant DNA technology and mRNA vaccines, is further propelling market growth. This innovative approach allows for the creation of safer, more effective, and easily administered vaccines, increasing market penetration and acceptance among veterinary professionals and pet owners. The rising prevalence of zoonotic diseases, emphasizing the interconnectedness of animal and human health, further underlines the importance of comprehensive vaccination programs and consequently contributes to the market's upward trajectory. Competitive pressures among major players, including Merck, Zoetis, and Boehringer Ingelheim, are stimulating innovation and affordability, making vaccines more accessible globally. This report comprehensively analyses this dynamic landscape, providing valuable insights into market trends, growth drivers, challenges, and future projections. The study period (2019-2033) covers both historical performance and future expectations, offering a complete picture of the veterinary biological vaccines market.
Several key factors are driving the expansion of the veterinary biological vaccines market. Firstly, the global rise in pet ownership is a significant contributor. More people are treating their pets as family members, leading to increased spending on their healthcare, including preventative measures like vaccinations. Secondly, growing awareness among pet owners and veterinarians regarding the importance of preventative healthcare, including vaccination, is pushing demand. This awareness is fostered through educational campaigns, veterinary consultations, and increased access to information. Thirdly, the continuous emergence of novel infectious diseases in animals necessitates the development and adoption of new vaccines to control outbreaks and prevent widespread infections. This necessitates ongoing research and development investment, supporting market growth. Furthermore, government initiatives and regulations supporting animal health and disease control programs often mandate vaccination, further boosting market demand. The development of more effective and safer vaccines, using advanced technologies, is also a strong driver. These new formulations lead to increased compliance and reduced adverse effects, making vaccination more appealing. Finally, the expanding veterinary services sector, with an increasing number of veterinary clinics and hospitals, provides wider access to vaccination services, directly impacting market reach and sales.
Despite the promising growth trajectory, the veterinary biological vaccines market faces certain challenges. High research and development costs associated with developing new vaccines and obtaining regulatory approvals can present a significant barrier to entry for smaller players, limiting innovation and potentially slowing market expansion. Furthermore, stringent regulatory approvals and compliance requirements across different countries pose an obstacle to the timely launch of new products, potentially delaying market penetration. The effectiveness of vaccines can vary based on several factors, including storage and handling conditions and the individual animal's immune response. Maintaining a consistent cold chain and ensuring proper vaccine administration are critical to guarantee efficacy, and any lapse can negatively affect the market perception. The presence of counterfeit or substandard vaccines in the market poses a significant threat to animal health and consumer trust. Robust measures to combat this illicit trade are needed to ensure the safety and efficacy of vaccines used. Additionally, consumer resistance to vaccination, driven by misinformation or concerns about potential side effects, remains a hurdle. Educational campaigns addressing these concerns are vital to improve vaccination uptake. Finally, economic factors, particularly in developing countries, can limit access to affordable vaccines, reducing market penetration in certain regions.
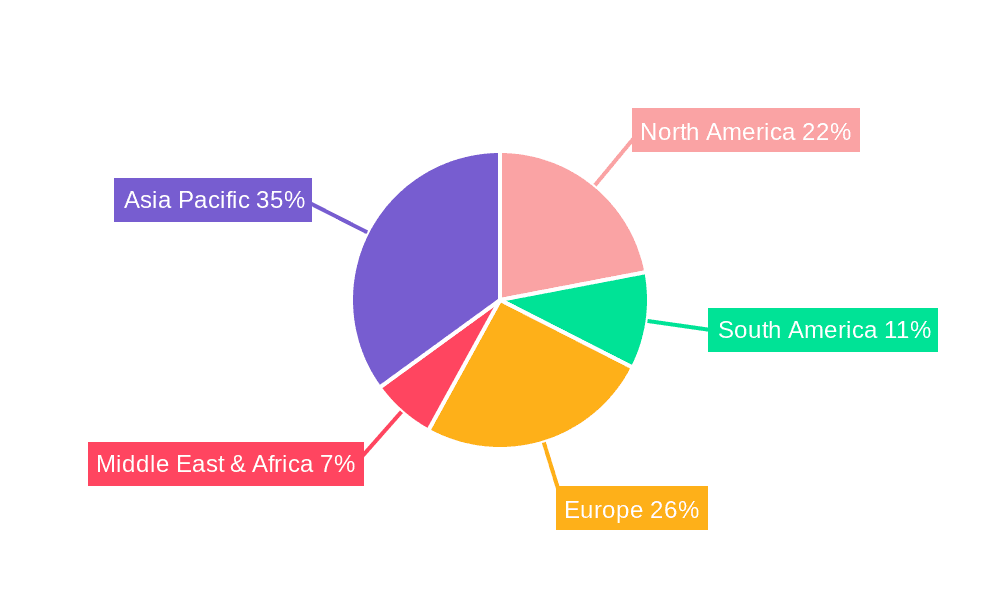
North America and Europe: These regions consistently demonstrate high per capita pet ownership and a well-established veterinary infrastructure, leading to high demand for veterinary biological vaccines. The strong regulatory framework and high disposable incomes further contribute to the dominance of these markets.
Asia-Pacific: This region exhibits rapid growth in pet ownership, particularly in developing economies like China and India. This burgeoning market presents a significant opportunity for expansion, albeit with challenges associated with infrastructure development and regulatory compliance.
Livestock Vaccines: The segment encompassing vaccines for livestock animals (cattle, poultry, swine) represents a substantial portion of the market due to the large-scale animal populations involved. This segment's expansion is driven by concerns about widespread disease outbreaks and the economic impact on agricultural sectors.
Paragraph Summary: The North American and European markets, characterized by high pet ownership rates and advanced veterinary services, are currently the dominant regions. However, the rapidly expanding pet ownership and livestock industries in the Asia-Pacific region are poised for significant growth, particularly in emerging economies. The livestock vaccine segment dominates due to the large-scale animal populations and the economic significance of disease prevention in the agricultural sector. The combination of these factors, including the growing awareness of animal health and the expansion of veterinary services, paints a picture of a diverse and dynamic market with substantial growth potential across various geographical regions and animal segments. The ongoing development of innovative vaccines, such as mRNA vaccines, will further contribute to this growth trajectory.
The veterinary biological vaccines industry is propelled by several key growth catalysts. Technological advancements resulting in more effective and safer vaccines, alongside rising pet ownership and increased veterinary care access, are primary drivers. Government regulations promoting animal health and disease control, coupled with rising awareness of preventative healthcare, further stimulate market expansion. The growing prevalence of zoonotic diseases underscores the crucial role of animal vaccination in safeguarding both animal and public health. The development of novel vaccines targeting emerging diseases and the incorporation of advanced delivery systems enhance market appeal and contribute to sustained growth.
(Note: Specific dates for these developments may need verification through publicly available sources. This list is illustrative and may not be exhaustive.)
This report offers an in-depth analysis of the veterinary biological vaccines market, providing a comprehensive overview of current trends, future projections, and key market players. It covers market size estimations, growth drivers, challenges, regional analysis, and competitive landscapes. The report’s detailed insights will be valuable for stakeholders across the industry, including manufacturers, distributors, and investors. It uses a combination of primary and secondary research methodologies, ensuring the accuracy and reliability of its data and analysis.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.8% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 5.8%.
Key companies in the market include Merck, Zoetis, Boehringer Ingelheim, Ceva, CAHIC, HVRI, Yebio, DHN, WINSUN, Elanco, Virbac, CAVAC, Kyoto Biken Laboratories, FATRO, Vaksindo, Bio-Labs, Avimex Animal Health, MEVAC, Biovac, Atafen, Dyntec, Ringpu Biology, Jinyu Bio-technology, Jinhe Biotechnology, China Animal Husbandry Industry, Wuhan Keqian Biology, Pulike Biological Engineering, Chengdu Kanghua Biological Products.
The market segments include Application, Type.
The market size is estimated to be USD 8252 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Veterinary Biological Vaccines," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Veterinary Biological Vaccines, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.