1. What is the projected Compound Annual Growth Rate (CAGR) of the Vaccine Particulate Adjuvants?
The projected CAGR is approximately 5%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Vaccine Particulate Adjuvants
Vaccine Particulate AdjuvantsVaccine Particulate Adjuvants by Type (Oral, Subcutaneous, Intranasal, Intramuscular, Intradermal, Others), by Application (Infectious Diseases, Cancer, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
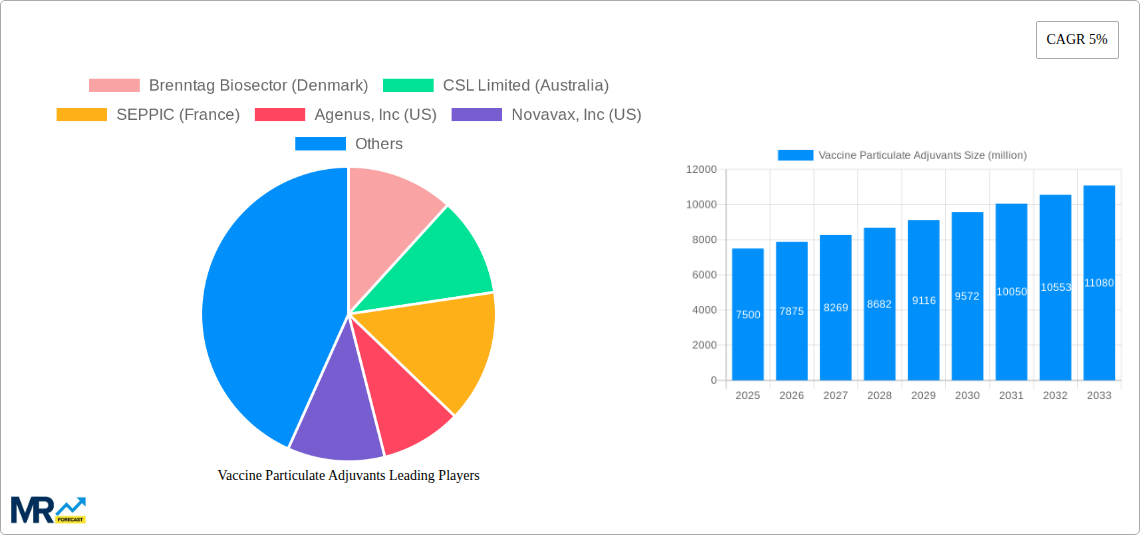
The Vaccine Particulate Adjuvants market is poised for significant expansion, projected to reach approximately $7,500 million by 2025, with a robust Compound Annual Growth Rate (CAGR) of 5% anticipated from 2025 through 2033. This growth is primarily propelled by the increasing demand for more potent and safer vaccine formulations, especially in combating infectious diseases and evolving cancer treatments. Particulate adjuvants, such as liposomes and nanoparticles, are crucial in enhancing the immune response to vaccines, leading to more effective and long-lasting protection. The rising prevalence of chronic and infectious diseases globally, coupled with substantial investments in research and development for novel vaccine technologies, are key market drivers. Furthermore, the growing awareness among healthcare providers and the public regarding the benefits of advanced adjuvant technologies is fueling market penetration. The trend towards personalized medicine and the development of sophisticated delivery systems for vaccines further underscore the importance of particulate adjuvants in achieving optimal therapeutic outcomes.
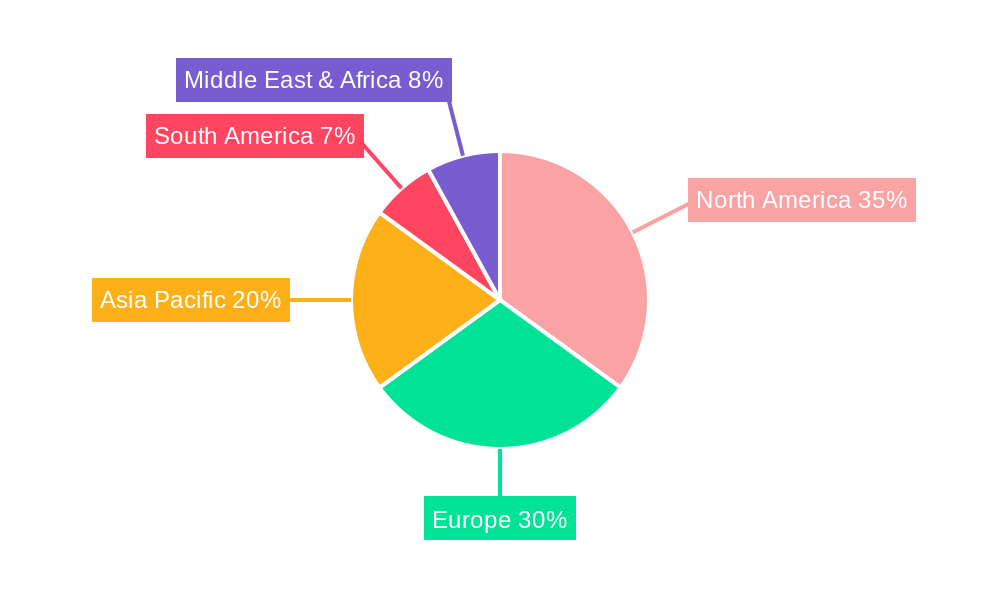
Despite the promising growth trajectory, certain restraints could influence market dynamics. High manufacturing costs associated with some advanced particulate adjuvants and stringent regulatory approval processes for new adjuvant formulations can pose challenges. However, ongoing technological advancements and innovations in production methods are expected to mitigate these concerns over time. The market is segmented by type, with Oral, Subcutaneous, and Intramuscular routes of administration representing significant segments due to their established efficacy and widespread use in vaccine delivery. In terms of application, Infectious Diseases and Cancer dominate the landscape, reflecting the urgent need for advanced immunizations against these critical health threats. Geographically, North America and Europe are expected to lead the market, driven by strong healthcare infrastructure, high R&D spending, and a proactive approach to vaccine development and adoption. The Asia Pacific region is also anticipated to witness substantial growth, fueled by a large population, increasing healthcare expenditure, and a growing focus on public health initiatives. Key players in this dynamic market include Brenntag Biosector, CSL Limited, and SEPPIC, among others, actively contributing to innovation and market expansion.
Here's a unique report description on Vaccine Particulate Adjuvants, incorporating the requested elements:
The global Vaccine Particulate Adjuvants market is experiencing a significant upswing, driven by a confluence of factors that underscore the evolving landscape of immunization. Over the Study Period of 2019-2033, and with a Base Year of 2025, this sector is poised for robust expansion. The Estimated Year of 2025 highlights the immediate positive outlook, building on the momentum from the Historical Period of 2019-2024. A key trend observed is the increasing sophistication of adjuvant technologies, moving beyond traditional aluminum salts to encompass a diverse array of particulate-based systems. These include liposomes, emulsions, polymeric nanoparticles, and mineral-based particles, each offering unique mechanisms for antigen presentation and immune system stimulation.
The market's trajectory is significantly influenced by the escalating global demand for more effective and safer vaccines, particularly against emerging infectious diseases. The COVID-19 pandemic acted as a powerful catalyst, accelerating research and development in adjuvant technologies and highlighting their critical role in enhancing vaccine immunogenicity and durability. Furthermore, the growing focus on prophylactic and therapeutic vaccines for chronic diseases, such as cancer, is opening new avenues for particulate adjuvants. Their ability to deliver antigens in a controlled manner and elicit specific immune responses makes them ideal candidates for cancer immunotherapy. The market is also witnessing a rise in combination vaccines, where particulate adjuvants play a crucial role in ensuring the efficacy of multiple antigens within a single formulation. This trend is expected to gain further traction in the coming years, driven by the pursuit of streamlined vaccination schedules and improved patient compliance. Innovations in material science and drug delivery are also contributing to the development of novel particulate adjuvants with enhanced targeting capabilities and reduced reactogenicity, further shaping the market's future.
The vaccine particulate adjuvants market is being propelled by several intertwined forces that promise sustained growth. A primary driver is the increasing global burden of infectious diseases, coupled with the emergence of novel pathogens, necessitating the rapid development and deployment of highly effective vaccines. Particulate adjuvants play a pivotal role in this by enhancing the immune response to vaccine antigens, leading to improved protection and reduced disease incidence. The growing prevalence of chronic diseases, such as cancer, and the significant advancements in cancer immunotherapy research have also emerged as major catalysts. Particulate adjuvants are instrumental in delivering tumor-associated antigens and stimulating anti-tumor immune responses, thereby opening new therapeutic frontiers.
Furthermore, the push towards developing next-generation vaccines with enhanced immunogenicity, longer-lasting immunity, and improved safety profiles is significantly fueling the demand for advanced particulate adjuvant technologies. Traditional adjuvants, while effective, can sometimes be associated with localized reactions. The innovation in particulate adjuvants aims to mitigate these concerns by offering more controlled antigen release and targeted immune cell activation. The increasing investments in research and development by both pharmaceutical companies and academic institutions, coupled with supportive government initiatives and funding for vaccine research, are also contributing to the accelerating pace of innovation and market expansion. The ongoing refinement of manufacturing processes for these complex adjuvants is also making them more accessible and scalable.
Despite the promising outlook, the vaccine particulate adjuvants market faces certain challenges and restraints that could temper its growth trajectory. A significant hurdle is the complexity and cost associated with the research, development, and manufacturing of novel particulate adjuvants. These advanced materials often require intricate synthesis processes, sophisticated characterization techniques, and stringent quality control measures, which can translate into higher production costs and longer development timelines. The regulatory approval process for new adjuvants can also be lengthy and demanding, requiring extensive preclinical and clinical studies to demonstrate safety and efficacy.
Another challenge lies in the potential for immunogenicity and reactogenicity. While particulate adjuvants are designed to enhance immune responses, some formulations can elicit undesirable local or systemic reactions, necessitating careful optimization to balance efficacy with tolerability. The variability in immune responses across different individuals and populations can also pose a challenge in predicting the universal effectiveness of certain adjuvant formulations. Furthermore, public perception and vaccine hesitancy can indirectly impact the market. Concerns surrounding the safety of novel vaccine components, including adjuvants, can lead to resistance to vaccination, thereby affecting market demand. The intellectual property landscape surrounding adjuvant technologies is also highly competitive, with multiple players vying for patent protection, which can create barriers to entry for new companies and limit the widespread adoption of certain technologies.
The Vaccine Particulate Adjuvants market is projected to witness significant dominance from North America and the Intramuscular segment, with Infectious Diseases as the leading application.
North America:
Intramuscular Segment:
Infectious Diseases Application:
The vaccine particulate adjuvants industry is experiencing significant growth driven by the relentless pursuit of enhanced vaccine efficacy and durability. The increasing prevalence of infectious diseases, including the emergence of novel pathogens, fuels the demand for more potent adjuvants. Furthermore, advancements in cancer immunotherapy are creating a burgeoning market for particulate adjuvants capable of eliciting targeted anti-tumor immune responses. The growing investment in research and development, coupled with supportive government initiatives and favorable regulatory pathways for innovative vaccine technologies, acts as a major growth catalyst. The continuous evolution of material science enabling the creation of novel particulate structures with improved antigen delivery and immune modulation capabilities also propels the industry forward.
This report provides an in-depth analysis of the global vaccine particulate adjuvants market, offering a comprehensive overview from 2019-2033. The study includes detailed market segmentation by Type (Oral, Subcutaneous, Intranasal, Intramuscular, Intradermal, Others), Application (Infectious Diseases, Cancer, Others), and geographic regions. With the Base Year of 2025 and an Estimated Year of 2025, the report meticulously analyzes market dynamics, including drivers, restraints, trends, and opportunities. It delves into Industry Developments, offering insights into technological advancements and their impact on market growth, alongside a thorough examination of the competitive landscape featuring key players. The forecast period of 2025-2033 provides strategic insights for stakeholders to navigate this dynamic and rapidly evolving market.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 5%.
Key companies in the market include Brenntag Biosector (Denmark), CSL Limited (Australia), SEPPIC (France), Agenus, Inc (US), Novavax, Inc (US), SPI Pharma, Inc (US), Invivogen (US), Avanti Polar Lipids, Inc (US), MVP Laboratories, Inc (US), OZ Biosciences (France), .
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Vaccine Particulate Adjuvants," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Vaccine Particulate Adjuvants, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.