1. What is the projected Compound Annual Growth Rate (CAGR) of the U.S. Real World Evidence Solutions Market?
The projected CAGR is approximately 11.6%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 U.S. Real World Evidence Solutions Market
U.S. Real World Evidence Solutions MarketU.S. Real World Evidence Solutions Market by Type (Clinical Setting Data, Claims Data, Pharmacy Data, Patient Powered Data, Others), by End-user (Pharmaceutical, Medical Device Companies, Healthcare Payers, Healthcare Providers, Others), by Forecast 2026-2034
The U.S. Real World Evidence Solutions Market size was valued at USD 6.10 USD Billion in 2023 and is projected to reach USD 13.15 USD Billion by 2032, exhibiting a CAGR of 11.6 % during the forecast period. Real World Evidence (RWE) Solutions stand for the use of methodologies based on data from the real world to provide results concerning the safety, effectiveness, and economic impacts of medical applications. Examples include EHRs, patient registries, and wearable devices that will be the key source data. Functions contain the implementation of an information security policy, data processing, and international regulations. For example, applications can include comparative effectiveness research, post-raid 1 subgenera surgery syndrome withdrawal marking surveillance, and adjustments during therapy. The demand for RWE solutions in the USA is on the rise for their capacity to complement clinical trial data, increase research effectiveness, and make evidence-based decisions in healthcare all while satisfying regulatory requirements set out by the country.
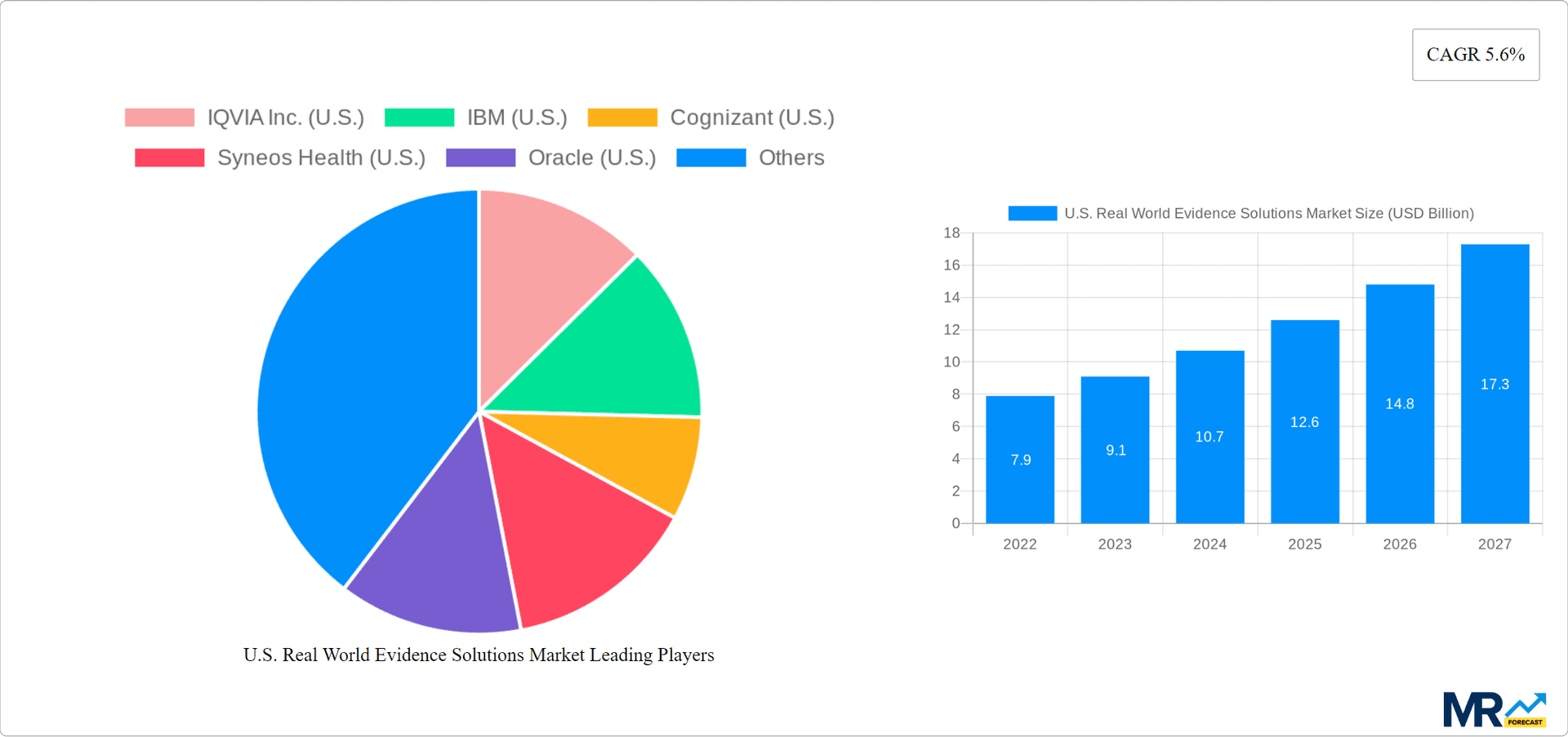

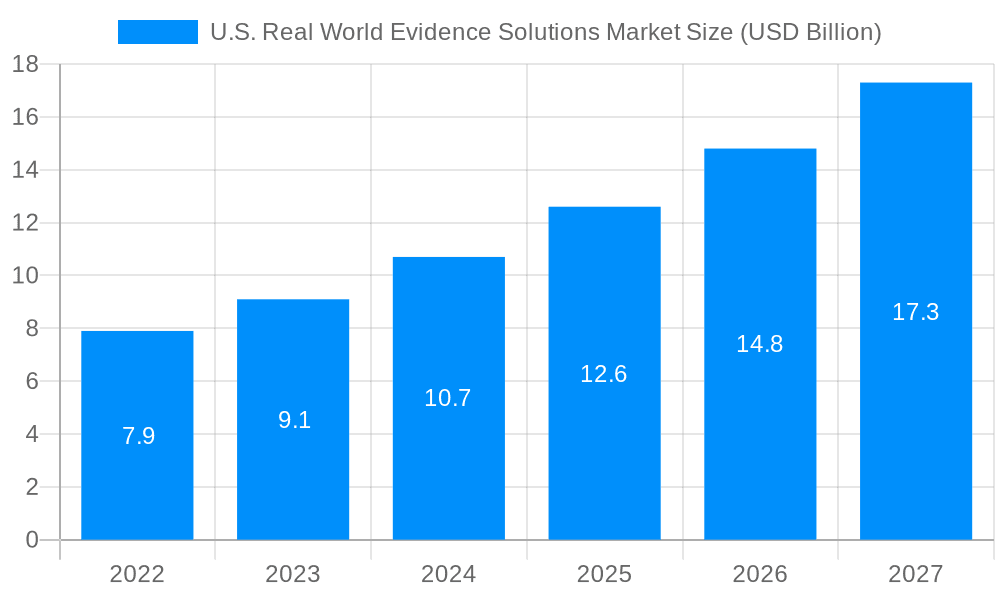

The U.S. is the largest market for RWE solutions, accounting for over 50% of the global market. The growth of the market in the U.S. is driven by the increasing demand for RWD to support regulatory decision-making, the rising adoption of value-based care models, and the growing focus on patient-centricity.

The pricing of RWE solutions varies depending on the type of data, the size of the study, and the complexity of the analysis. However, the average cost of an RWE study is between USD 100,000 and USD 1 million.
The U.S. is a net importer of RWE solutions. The major import sources include the European Union, Canada, and Japan.
Type:
End-user:
There are a number of patents and trademarks related to RWE solutions. These include patents for methods of collecting, analyzing, and interpreting RWD, as well as trademarks for RWE solution products and services.
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 11.6% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 11.6%.
Key companies in the market include IQVIA Inc. (U.S.), IBM (U.S.), Cognizant (U.S.), Syneos Health (U.S.), Oracle (U.S.), Optum Inc. (United Health Group) (U.S.), Flatiron Health (F. Hoffmann-La Roche) (U.S.), Thermo Fisher Scientific Inc. (U.S.), Parexel International Corporation (U.S.), SAS Institute Inc. (U.S.), Perkin Elmer Inc. (U.S.).
The market segments include Type, End-user.
The market size is estimated to be USD 13.15 USD Billion as of 2022.
Increasing Usage of Real World Evidence Solutions by Biopharmaceutical Companies to Propel the Demand for Preventive Products.
Increasing Adoption of Value Based Healthcare.
Challenges Associated with Data Quality and Privacy to Hinder Market Growth.
May 2023: Flatiron Health expanded its RWE offerings to include scientific, analytical, and consulting services.
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 2850, USD 3850, and USD 4850 respectively.
The market size is provided in terms of value, measured in USD Billion.
Yes, the market keyword associated with the report is "U.S. Real World Evidence Solutions Market," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the U.S. Real World Evidence Solutions Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.