1. What is the projected Compound Annual Growth Rate (CAGR) of the Sterile and Antiviral Packaging?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Sterile and Antiviral Packaging
Sterile and Antiviral PackagingSterile and Antiviral Packaging by Type (Hard Plastic, Flexible Material, Other), by Application (Drug, Health Care, Other), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The Sterile and Antiviral Packaging market is poised for substantial expansion, driven by increasing global health consciousness and the persistent demand for enhanced product safety, particularly within the pharmaceutical and healthcare sectors. With a current market size estimated at approximately $5,500 million and projected to grow at a Compound Annual Growth Rate (CAGR) of roughly 8%, the market is set to reach an estimated $11,500 million by 2033. This growth is fueled by escalating concerns over hospital-acquired infections and the need for robust solutions to prevent microbial contamination in sensitive medical devices, drugs, and diagnostic kits. The rising prevalence of chronic diseases and an aging global population further amplify the demand for sterile packaging that ensures the integrity and efficacy of life-saving medications and treatments. Advancements in material science, leading to the development of more effective antiviral and antimicrobial additives and coatings, are also significant contributors to market expansion. Innovations in packaging technologies, such as active packaging that actively inhibits microbial growth and smart packaging with indicators for sterility, are shaping the future landscape of this vital market.
The market is segmented by material type, with Hard Plastic packaging likely dominating due to its durability, barrier properties, and cost-effectiveness for a wide range of applications. Flexible materials, however, are gaining traction for their lightweight nature and recyclability, especially in specific drug delivery systems. In terms of application, the Drug segment is the primary revenue generator, followed closely by Health Care. The increasing stringency of regulatory requirements globally, mandating higher standards for sterile and antiviral packaging, further acts as a significant driver. Despite these positive trends, market restraints include the higher cost associated with advanced antiviral materials and the complex manufacturing processes required. Furthermore, the potential for resistance development in microbes against certain antiviral agents necessitates continuous research and development, which can add to operational costs. Key industry players like Avery Dennison Corporation, E.l.du Pont de Nemours, and CCL Industries Inc. are actively investing in R&D to offer innovative solutions and maintain a competitive edge in this dynamic and critical market.
Here is a unique report description on Sterile and Antiviral Packaging, incorporating your specified requirements:
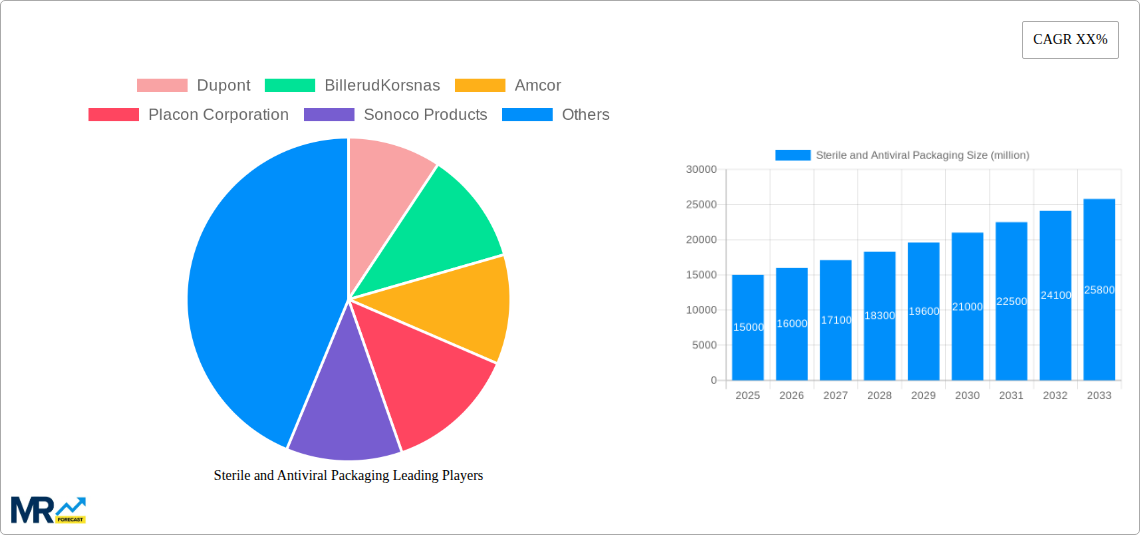
The global sterile and antiviral packaging market is poised for substantial growth, driven by an intensified focus on public health and a heightened awareness of contamination risks. During the historical period of 2019-2024, the market experienced a steady upward trajectory, fueled by the immediate demands of the COVID-19 pandemic, which underscored the critical role of packaging in preventing disease transmission. This surge in demand saw the market reach approximately 15,000 million units in the base year of 2025. Looking ahead, the forecast period of 2025-2033, with the estimated year of 2025 serving as a key benchmark, predicts an impressive compound annual growth rate (CAGR). This growth is not merely a temporary blip but a sustained evolution driven by fundamental shifts in consumer behavior and industry practices. The estimated market size is projected to ascend to over 35,000 million units by 2033, reflecting a significant expansion.
Key market insights reveal a nuanced landscape. The Drug application segment is expected to maintain its dominance throughout the study period (2019-2033), accounting for a substantial share of the market. This is attributed to the stringent regulatory requirements for pharmaceutical products, demanding unwavering sterility and protection from external contaminants. Simultaneously, the Health Care segment, encompassing medical devices, diagnostic kits, and personal protective equipment, is also witnessing robust expansion. The increasing prevalence of chronic diseases and the growing need for advanced medical interventions further bolster this segment.
Furthermore, advancements in material science are continuously shaping the market. The Flexible Material type, offering versatility, cost-effectiveness, and enhanced barrier properties, is gaining considerable traction. Innovations in films, pouches, and sachets incorporating antimicrobial agents are becoming increasingly prevalent, providing an additional layer of defense. The Hard Plastic segment, while established, is also evolving with the integration of antimicrobial additives and improved sealing technologies to meet sterilization requirements. The "Other" category, which encompasses specialized packaging solutions for sensitive biotechnological products and advanced wound care, is also expected to exhibit strong growth, albeit from a smaller base. The overarching trend points towards a more sophisticated and multi-layered approach to packaging, where sterility assurance and active antiviral properties are no longer optional but essential.
The escalating global demand for sterile and antiviral packaging is being propelled by a confluence of powerful forces. Paramount among these is the heightened public and regulatory scrutiny on hygiene and contamination control, a trend significantly amplified by the recent global health crisis. This has instilled a deep-seated awareness, extending beyond healthcare settings to everyday consumer products, demanding greater assurance of sterility and protection from harmful pathogens. Industry-specific regulations, particularly within the pharmaceutical and healthcare sectors, continue to tighten, mandating the use of packaging solutions that guarantee the integrity and sterility of sensitive products throughout their lifecycle.
The rapid pace of innovation in material science and packaging technologies plays a crucial role. Manufacturers are actively developing and integrating novel antimicrobial agents and barrier technologies into packaging materials, offering enhanced efficacy and extended shelf life. This includes advancements in biodegradable and sustainable antiviral packaging solutions, aligning with growing environmental consciousness. Furthermore, the expanding pharmaceutical and biotechnology industries, with their continuous introduction of new drugs, vaccines, and complex medical devices, inherently require sophisticated sterile packaging to maintain product efficacy and patient safety. The increasing global healthcare expenditure and the growing accessibility of medical treatments, especially in emerging economies, further contribute to the sustained demand for reliable sterile packaging. The perceived value of enhanced protection for consumers and healthcare professionals alike is a powerful psychological driver, encouraging the adoption of antiviral packaging solutions across a wider range of applications.
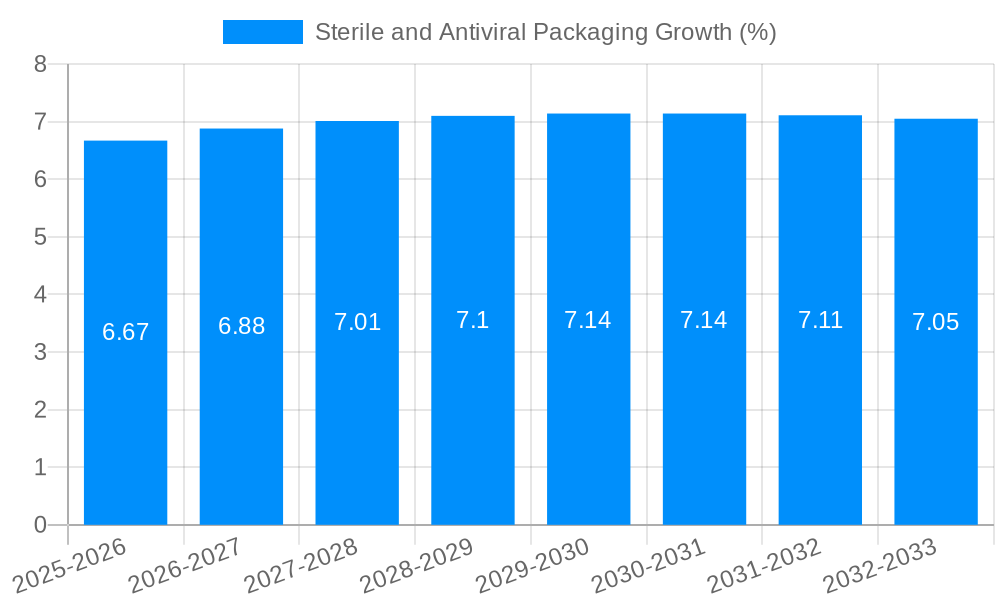
Despite the robust growth trajectory, the sterile and antiviral packaging market faces several significant challenges and restraints that could temper its expansion. A primary hurdle lies in the cost of implementation and production. Advanced antiviral additives and sophisticated sterilization processes can significantly increase the manufacturing costs of packaging materials, potentially impacting their affordability and adoption, especially for price-sensitive markets or lower-value products. This cost factor can be particularly challenging for smaller enterprises with limited R&D budgets and capital investment capabilities.
Another considerable challenge is regulatory complexity and evolving standards. While regulations drive demand, navigating the diverse and often intricate regulatory landscapes across different countries and regions can be a daunting task for manufacturers. Obtaining necessary approvals and certifications for antiviral packaging, especially concerning the efficacy and safety of the antimicrobial agents used, requires substantial investment in testing and documentation. Furthermore, the potential for antimicrobial resistance is a growing concern. Over-reliance on antimicrobial packaging without proper understanding of their mechanisms and potential for resistance development could lead to unintended consequences, necessitating careful research and responsible product development.
The limited shelf-life of some antiviral properties in packaging can also pose a restraint. While innovations are constantly being made, the long-term efficacy of embedded antiviral agents can be compromised by factors such as exposure to light, heat, or other environmental conditions. This necessitates continuous research to improve the durability and stability of these properties. Finally, consumer perception and education regarding the necessity and safety of antiviral packaging remain areas that require ongoing attention. Misconceptions or a lack of understanding about the benefits and functionality of such packaging can hinder widespread consumer acceptance and drive demand.
The Drug application segment is anticipated to be the dominant force in the sterile and antiviral packaging market throughout the study period (2019-2033), with its estimated market size in 2025 reaching approximately 9,000 million units. This segment's leadership is underpinned by the critical need to maintain the sterility and integrity of pharmaceuticals, from life-saving medications to over-the-counter drugs. The stringent regulatory requirements imposed by bodies like the FDA and EMA necessitate advanced packaging solutions that prevent microbial contamination, degradation, and cross-contamination. The increasing global demand for pharmaceuticals, driven by an aging population, rising chronic disease rates, and advancements in drug discovery, further solidifies the Drug segment's market dominance.
Within this segment, innovations in flexible packaging solutions, such as sterile pouches, blister packs with enhanced barrier properties, and tamper-evident seals, are particularly noteworthy. These materials offer a combination of protection, convenience, and cost-effectiveness. The development of advanced multi-layer films incorporating antimicrobial agents and oxygen/moisture barriers is crucial for extending the shelf life and ensuring the efficacy of sensitive drug formulations.
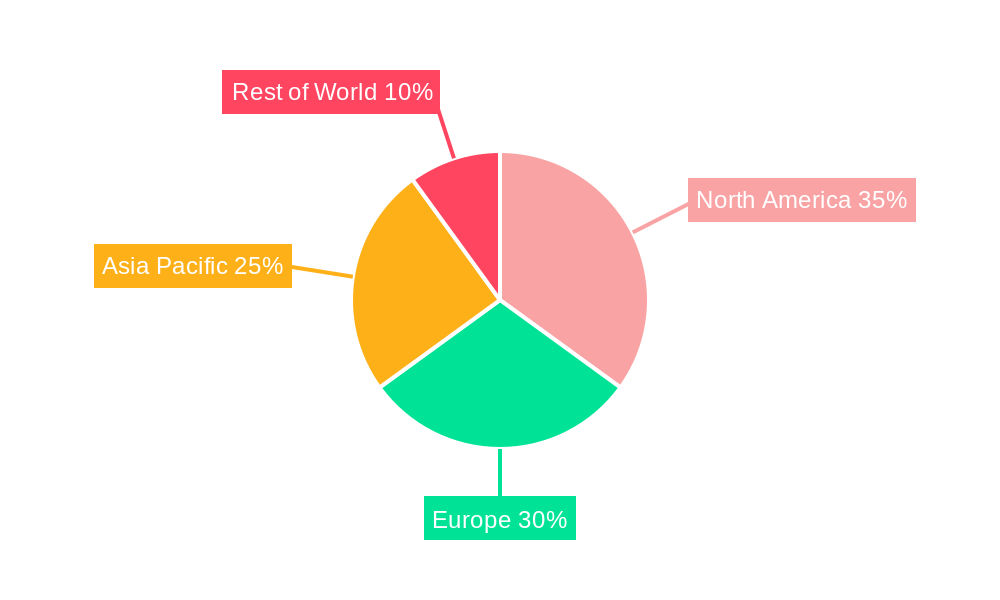
Geographically, North America and Europe are expected to continue their reign as the leading regions in the sterile and antiviral packaging market. North America, with its robust pharmaceutical industry, advanced healthcare infrastructure, and high disposable incomes, exhibits a strong demand for high-quality, sterile packaging solutions. The region's proactive regulatory environment and significant investment in R&D contribute to its market leadership, with an estimated market share of around 35% in 2025, valued at approximately 5,250 million units.
Europe follows closely, driven by similar factors, including a well-established pharmaceutical sector and a strong emphasis on patient safety. The stringent quality standards and the increasing focus on preventing healthcare-associated infections (HAIs) in this region further bolster the demand for sterile and antiviral packaging. The collective market value of these two regions in 2025 is estimated to be well over 10,000 million units.
The Health Care segment, while secondary to Drugs, is also a significant contributor, encompassing medical devices, diagnostic kits, and hospital supplies. The increasing global focus on infection control and the growing demand for sophisticated medical devices are fueling growth in this segment, with an estimated market size of around 4,500 million units in 2025. Innovations in this segment include sterile barrier systems for surgical instruments, antimicrobial coatings for medical devices, and specialized packaging for in-vitro diagnostic kits.
The sterile and antiviral packaging industry is experiencing significant growth catalysts that are shaping its future. The ongoing global emphasis on public health and hygiene, significantly amplified by recent pandemics, continues to be a primary driver, fostering a sustained demand for packaging that actively prevents pathogen transmission. Advancements in material science, leading to the development of novel antimicrobial additives, self-sanitizing surfaces, and enhanced barrier properties, are creating more effective and diverse packaging solutions. The expanding pharmaceutical and biotechnology sectors, with their continuous pipeline of new drugs and medical devices requiring stringent sterility, provide a consistent market for these advanced packaging materials. Furthermore, increasing healthcare expenditure globally, particularly in emerging economies, is translating into a greater demand for safe and sterile medical products, thereby fueling the need for reliable sterile and antiviral packaging.
This comprehensive report on the sterile and antiviral packaging market offers an in-depth analysis of market dynamics, trends, and growth opportunities. It provides detailed insights into the market's evolution from 2019 to 2033, with a foundational base year of 2025 and a forecast period extending to 2033. The report delves into the critical driving forces, such as heightened public health awareness and stringent regulatory demands, that are propelling the market forward. Simultaneously, it critically examines the challenges and restraints, including cost considerations and regulatory complexities, that could influence market expansion. The report identifies key regions and dominant segments, such as the Drug application and North America, offering detailed market size estimations in million units for 2025 and projections for the forecast period. It further elaborates on the growth catalysts, leading players, and significant developments shaping the industry. This report is designed to provide stakeholders with the essential information needed to navigate and capitalize on the burgeoning sterile and antiviral packaging landscape.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Avery Dennison Corporation, E.l.du Pont de Nemours, CCL Industries Inc, BioCote, Avient, Mondi, Biomaster, BASF, Lonza, Takex Labo Co.Ltd, Berry inc..
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Sterile and Antiviral Packaging," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Sterile and Antiviral Packaging, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.