1. What is the projected Compound Annual Growth Rate (CAGR) of the Short-acting Recombinant Human Erythropoietin?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Short-acting Recombinant Human Erythropoietin
Short-acting Recombinant Human ErythropoietinShort-acting Recombinant Human Erythropoietin by Type (Epoetin-alfa, Epoetin-beta, Darbepoetin-alfa, Others, World Short-acting Recombinant Human Erythropoietin Production ), by Application (Anemia, Kidney Disorders, Others, World Short-acting Recombinant Human Erythropoietin Production ), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The global market for Short-acting Recombinant Human Erythropoietin (rhEPO) is poised for substantial growth, currently valued at an estimated $14,120 million. This expansion is driven by a confluence of factors, including the increasing prevalence of anemia, particularly among patients with chronic kidney disease (CKD) and those undergoing chemotherapy. The growing demand for effective treatments to stimulate red blood cell production, coupled with advancements in biotechnology leading to improved rhEPO formulations, are key catalysts. The market is segmented by type, with Epoetin-alfa and Epoetin-beta holding significant shares due to their established efficacy and widespread use. Darbepoetin-alfa, a longer-acting analogue, also contributes to market dynamics, offering alternative treatment regimens. The application landscape is dominated by anemia and kidney disorders, reflecting the primary therapeutic areas for rhEPO. Emerging economies, with their expanding healthcare infrastructure and rising awareness of anemia management, present significant untapped potential.
The projected Compound Annual Growth Rate (CAGR) for the Short-acting rhEPO market is robust, indicating a sustained upward trajectory. Key market players, including Amgen, Johnson & Johnson, and Roche, are investing heavily in research and development to enhance product pipelines and expand market reach through strategic collaborations and geographical expansions. While the market is generally optimistic, potential restraints include stringent regulatory approvals for new products, the development of biosimilars, and increasing cost-containment pressures within healthcare systems. However, the persistent need for blood transfusions in various medical conditions and the inherent advantages of rhEPO in improving patient quality of life are expected to outweigh these challenges, ensuring continued market expansion through 2033.
Here is a unique report description for Short-acting Recombinant Human Erythropoietin, incorporating your specified requirements:
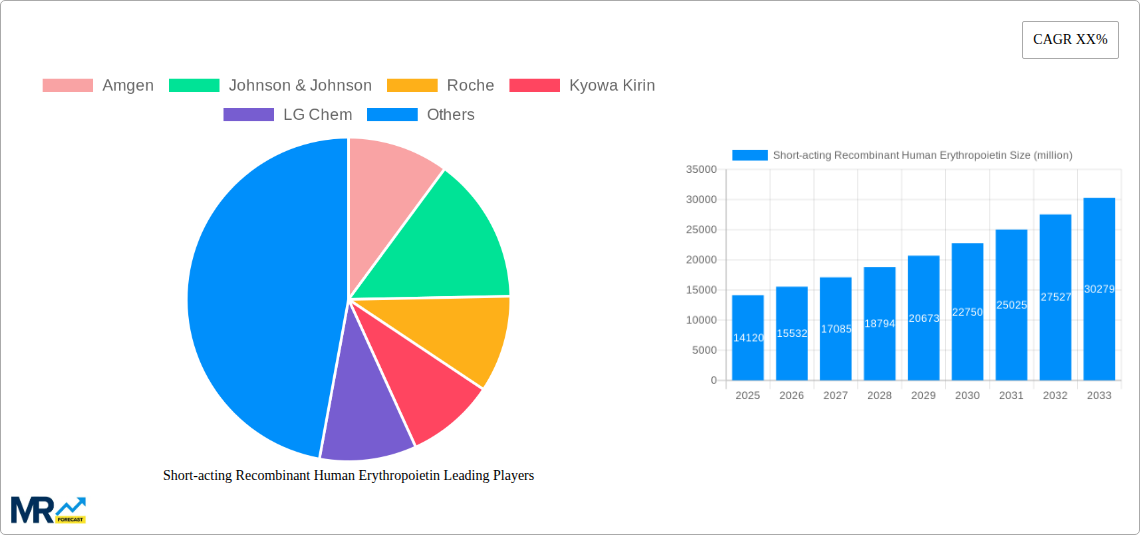
The global Short-acting Recombinant Human Erythropoietin (rhEPO) market is poised for a dynamic evolution, projected to witness significant growth and strategic shifts throughout the study period of 2019-2033, with the estimated year of 2025 serving as a pivotal point for current market assessments and a springboard for future forecasts. This segment of the biopharmaceutical industry, crucial for addressing anemias and related conditions, is characterized by a complex interplay of established players and emerging innovators. The market is expected to surge from its historical performance during 2019-2024, driven by an expanding patient base, advancements in production technologies, and an increasing awareness of rhEPO's therapeutic benefits. The estimated market size, measured in millions of units, is anticipated to demonstrate a robust upward trajectory, reflecting the sustained demand for effective treatments. Key trends include the ongoing refinement of manufacturing processes to enhance purity and yield, leading to potentially more cost-effective production of these life-saving biologics. Furthermore, the competitive landscape is continually shaped by patent expirations and the subsequent rise of biosimilars, which are expected to broaden market access and affordability, particularly in emerging economies. The adoption of advanced analytical techniques for quality control and characterization is also a significant trend, ensuring the safety and efficacy of rhEPO products. The intrinsic value of rhEPO in improving quality of life for patients with chronic kidney disease (CKD) and other debilitating conditions underpins its consistent market relevance. The market's growth will also be influenced by regulatory landscapes and the ongoing efforts of healthcare systems to manage the burden of anemia, making rhEPO a vital therapeutic option. The increasing focus on personalized medicine might also introduce nuances in rhEPO application, albeit its primary role remains in addressing generalized erythropoietic deficiency.
Several potent forces are collectively propelling the growth and expansion of the Short-acting Recombinant Human Erythropoietin market. Foremost among these is the escalating global prevalence of chronic kidney disease (CKD), a primary indication for rhEPO therapy. As populations age and lifestyle-related conditions like diabetes and hypertension rise, the incidence of CKD is projected to continue its upward trend, directly translating into a greater demand for rhEPO to manage associated anemia. Furthermore, advancements in oncological treatments, particularly chemotherapy, often induce anemia as a significant side effect. The increasing sophistication and widespread use of chemotherapy regimens globally necessitate effective countermeasures, with rhEPO playing a critical role in mitigating treatment-induced anemia, improving patient tolerance, and maintaining treatment protocols. Beyond these core applications, there's a growing recognition of rhEPO's potential in other clinical scenarios where anemia is a contributing factor to morbidity, such as in certain inflammatory conditions or pre-operative anemia management. The continuous innovation in recombinant DNA technology and bioprocessing techniques employed by leading pharmaceutical companies is also a significant driver. These advancements lead to improved production efficiencies, higher product yields, and enhanced product quality, ultimately making rhEPO more accessible and reliable for patients. The global healthcare infrastructure's increasing capacity, particularly in developing nations, and a greater emphasis on evidence-based medicine further support the market's expansion by ensuring broader patient access to these vital treatments.
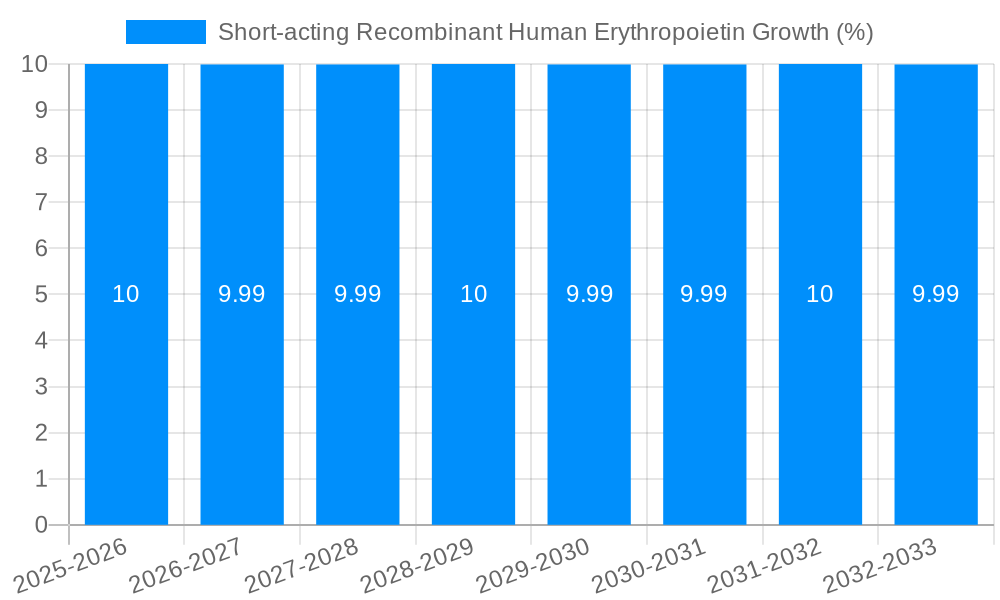
Despite its significant therapeutic importance, the Short-acting Recombinant Human Erythropoietin market is not without its challenges and restraints, which could temper its growth trajectory. A primary concern remains the significant cost associated with the production and administration of rhEPO. While biosimilars are emerging, the initial investment in research, development, and sophisticated manufacturing processes for biologics is substantial, often leading to high per-unit costs that can strain healthcare budgets, especially in resource-limited settings. This cost factor can also lead to access issues for a significant portion of the patient population globally. Regulatory hurdles, though necessary for ensuring safety and efficacy, can also present a challenge. The stringent approval processes for biologics, coupled with evolving guidelines and pharmacovigilance requirements, can prolong time-to-market for new rhEPO products and require substantial compliance investments from manufacturers. Moreover, concerns regarding the long-term safety profile of rhEPO, particularly in certain patient populations and at supra-physiological doses, have led to cautious prescribing practices and the establishment of strict dosing guidelines. Adverse events, such as thromboembolic events, have been associated with rhEPO therapy, necessitating careful patient selection and monitoring. The emergence and increasing acceptance of alternative therapeutic strategies for anemia management, such as iron supplementation and novel oral iron chelators, could also present a competitive restraint by offering less expensive or more convenient treatment options for specific patient profiles. Finally, the patent landscape surrounding established rhEPO products can influence market dynamics, with generic competition posing a threat to originator revenues once patents expire.
The global Short-acting Recombinant Human Erythropoietin market's dominance will be multifaceted, influenced by both regional healthcare infrastructures and specific product segments.
Dominating Segments:
Type: Epoetin-alfa & Epoetin-beta:
Application: Kidney Disorders:
Dominating Region/Country:
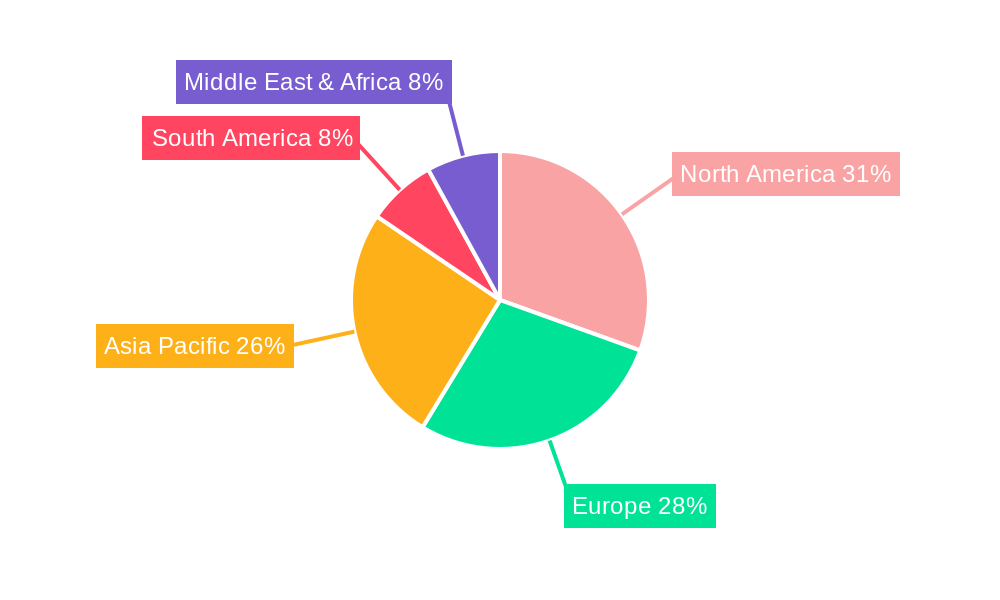
North America (Primarily the United States):
Europe:
Asia-Pacific (with a focus on China and Japan):
The Short-acting Recombinant Human Erythropoietin industry is propelled by several key growth catalysts. The escalating global incidence of chronic kidney disease (CKD) and the widespread use of chemotherapy in oncology are primary drivers, directly increasing the patient population requiring erythropoiesis stimulation. Furthermore, advancements in biosimilar development are expanding market access and affordability, particularly in emerging economies, by offering cost-effective alternatives. Continuous innovation in biomanufacturing processes also contributes by enhancing production efficiency and product quality. Growing physician awareness and patient education regarding the benefits of rhEPO in managing anemia-related fatigue and improving quality of life further stimulate demand.
This comprehensive report offers an in-depth analysis of the Short-acting Recombinant Human Erythropoietin market, spanning the historical period of 2019-2024 and projecting future trends up to 2033. It meticulously examines market drivers, restraints, and opportunities, providing a granular view of segment-specific performance, including Epoetin-alfa, Epoetin-beta, and Darbepoetin-alfa, across key applications like Anemia and Kidney Disorders. The report delves into regional market dynamics, identifying dominant countries and continents, with a particular focus on production capacities and market penetration strategies of leading players. It also highlights significant industry developments, regulatory landscapes, and emerging technological advancements that will shape the market's trajectory. With detailed market sizing, segmentation, and forecasting, this report serves as an indispensable resource for stakeholders seeking to understand and capitalize on the evolving global rhEPO market.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Amgen, Johnson & Johnson, Roche, Kyowa Kirin, LG Chem, Daewoong Pharmaceutical, Wockhardt, CJ Healthcare, 3SBio, Kexing Biopharm, Harbin Pharmaceutical Group, Beijing Four Rings Bio-Pharmaceutical, .
The market segments include Type, Application.
The market size is estimated to be USD 14120 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Short-acting Recombinant Human Erythropoietin," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Short-acting Recombinant Human Erythropoietin, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.