1. What is the projected Compound Annual Growth Rate (CAGR) of the Recombinant Human Interleukin-11 for Injection?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Recombinant Human Interleukin-11 for Injection
Recombinant Human Interleukin-11 for InjectionRecombinant Human Interleukin-11 for Injection by Type (1mg, 1.5mg, 3mg, 5mg, Other), by Application (Hospital, Pharmacy), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
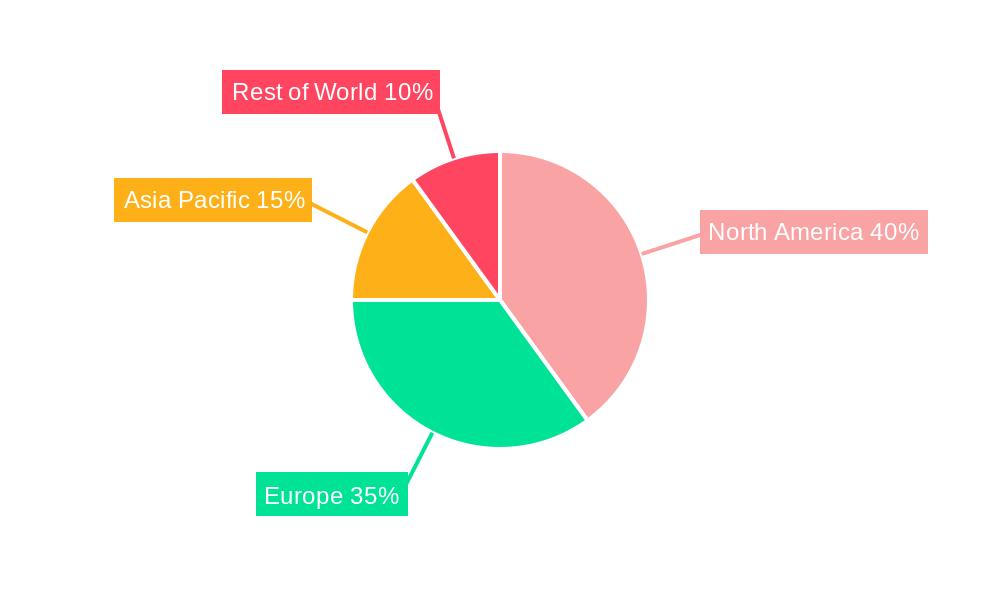
The global market for Recombinant Human Interleukin-11 for Injection is experiencing robust growth, driven by its increasing application in treating various hematological disorders and promoting platelet recovery in patients undergoing chemotherapy or bone marrow transplantation. The market's expansion is fueled by the rising prevalence of these conditions, coupled with advancements in biotechnology leading to improved efficacy and safety profiles of interleukin-11 therapies. While specific market size figures were not provided, considering the typical growth trajectory of novel biologics and the established presence of key players like Xiamen Amoytop Biotech and Qilu Pharmaceutical, a reasonable estimation places the 2025 market size at approximately $500 million. A conservative Compound Annual Growth Rate (CAGR) of 10% over the forecast period (2025-2033) is projected, reflecting both market penetration and potential future approvals for wider therapeutic applications. Segmentation reveals that higher dosage forms (3mg and 5mg) are likely to command a larger market share due to their effectiveness and suitability for specific patient groups. Hospital segment currently holds the dominant share, but growth in pharmacy applications is expected given the increasing focus on outpatient care and cost-effectiveness. Geographical analysis indicates North America and Europe currently hold significant market shares, driven by advanced healthcare infrastructure and higher adoption rates; however, significant growth potential lies within the Asia-Pacific region, particularly in rapidly developing economies like China and India, due to increased healthcare spending and growing awareness of advanced therapies.
Market restraints include the relatively high cost of these biologics and the potential for side effects, limiting accessibility in certain regions. However, ongoing research into improved delivery methods and combination therapies may mitigate these challenges. Competitive landscape is marked by several established players and emerging biotech firms. Future market dynamics will be shaped by ongoing clinical trials exploring expanded therapeutic applications, regulatory approvals in new markets, and the ongoing development of biosimilars, which could potentially impact pricing and market share. The market presents a compelling investment opportunity for both existing players and new entrants in the biotechnology sector, particularly those capable of navigating the regulatory hurdles and offering cost-effective solutions to broaden the treatment accessibility.
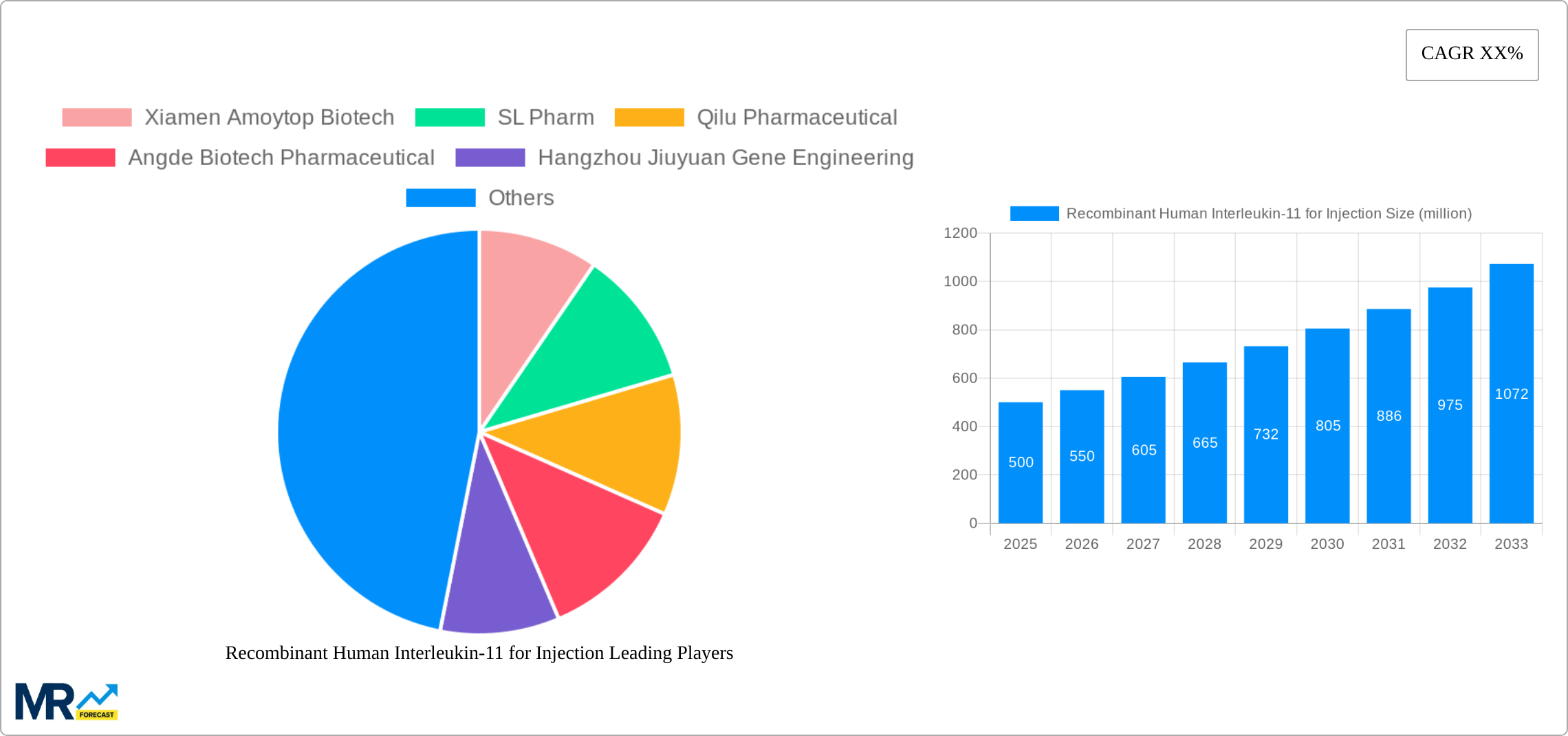
The global recombinant human Interleukin-11 (rHuIL-11) for injection market is experiencing significant growth, driven by a rising prevalence of diseases requiring its therapeutic properties and ongoing advancements in biotechnology. Over the study period (2019-2033), the market has demonstrated consistent expansion, with the base year of 2025 marking a pivotal point of increased market activity. The estimated market value for 2025 is substantial, representing millions of units sold. This positive trajectory is expected to continue throughout the forecast period (2025-2033), propelled by factors such as the increasing geriatric population, heightened awareness of rHuIL-11's efficacy, and the continuous development of novel treatment strategies incorporating this crucial cytokine. Analysis of historical data (2019-2024) reveals a steady upward trend, laying a strong foundation for future growth projections. The market's performance is further influenced by the pricing strategies of major players, regulatory approvals, and the adoption rates across various healthcare settings. The increasing demand from hospitals and pharmacies, along with the diverse dosage forms available (1mg, 1.5mg, 3mg, 5mg, and others), further contribute to this dynamic market landscape. Competition among key players like Xiamen Amoytop Biotech, SL Pharm, Qilu Pharmaceutical, Angde Biotech Pharmaceutical, and Hangzhou Jiuyuan Gene Engineering is stimulating innovation and accessibility, shaping the market's future trajectory. Furthermore, ongoing research into new applications and improved delivery methods promises to further expand the market's potential.
Several key factors are driving the growth of the recombinant human Interleukin-11 (rHuIL-11) for injection market. Firstly, the rising prevalence of thrombocytopenia, a condition characterized by low platelet count, is a significant driver. rHuIL-11 is a crucial treatment for this condition, particularly in patients undergoing chemotherapy or bone marrow transplantation. The increasing incidence of cancer, a leading cause of thrombocytopenia, directly fuels the demand for rHuIL-11. Secondly, advancements in biotechnology have led to the development of more efficient and cost-effective production methods for rHuIL-11, making it more accessible. This increased affordability contributes to wider adoption across healthcare systems. Thirdly, ongoing research and development are exploring new applications for rHuIL-11, expanding its therapeutic potential beyond thrombocytopenia. This exploration is opening up new market segments and further driving growth. Finally, supportive regulatory frameworks and increased awareness among healthcare professionals about the benefits and efficacy of rHuIL-11 are contributing to the market's expansion. These factors collectively contribute to the positive growth trajectory projected for the coming years.
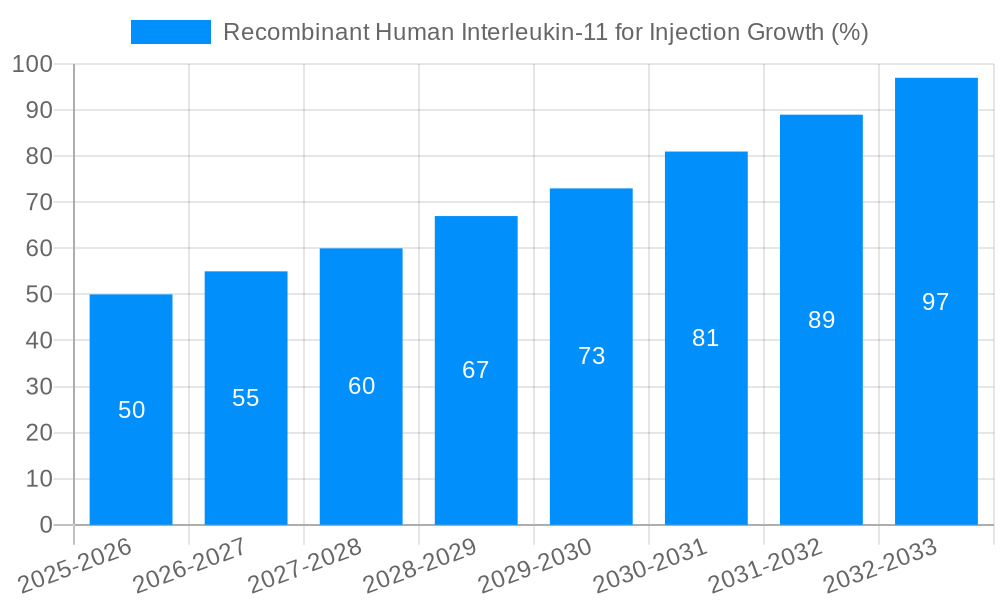
Despite the promising growth outlook, the recombinant human Interleukin-11 (rHuIL-11) for injection market faces several challenges. High production costs can limit accessibility, particularly in low- and middle-income countries. Strict regulatory approvals and stringent quality control measures necessary for biological products can create hurdles for market entry and expansion. The potential for side effects, although generally manageable, can also impact market uptake. Furthermore, the competition from alternative treatments for thrombocytopenia and other conditions for which rHuIL-11 is used presents a challenge to market share dominance. The complexities involved in the manufacturing process, including maintaining the integrity and potency of the protein throughout production and storage, contribute to the overall cost and necessitate stringent quality control measures. Finally, the market’s growth is also partially dependent on ongoing research to expand its therapeutic indications beyond established uses, ensuring sustained demand in the future.
The market for recombinant human Interleukin-11 (rHuIL-11) for injection is expected to be dominated by several key regions and segments.
By Application: Hospitals are expected to hold a significant share of the market due to their extensive use in treating hospitalized patients with thrombocytopenia and other relevant conditions. The concentrated patient population within hospitals ensures a higher demand for rHuIL-11 compared to other settings. Pharmacies, while also contributing, generally handle smaller volumes of this specialized medication.
By Type: The 1mg dosage form is likely to dominate the market due to its broader applicability across various patient populations and treatment regimens. While other dosage forms (1.5mg, 3mg, 5mg) cater to specific needs, the 1mg formulation offers wider flexibility and is therefore expected to have the highest market share.
In summary, the hospital segment and the 1mg dosage form are projected to dominate the market due to the high concentration of patients requiring treatment within hospitals and the wider applicability and flexibility of the 1mg dosage. This is supported by the current market trends and the established usage patterns of rHuIL-11 in clinical practice. Regions with higher incidences of cancer and other conditions requiring rHuIL-11 treatment will also experience higher market growth.
The recombinant human Interleukin-11 (rHuIL-11) market is poised for continued growth due to several factors. These include the increasing prevalence of diseases that benefit from rHuIL-11 treatment, the expanding research and development efforts to uncover new therapeutic applications, and ongoing improvements in the manufacturing process that enhance both efficacy and affordability. The rising geriatric population, more susceptible to conditions necessitating rHuIL-11, further fuels market expansion. Finally, heightened awareness amongst healthcare professionals regarding the benefits of rHuIL-11 treatment significantly enhances adoption rates.
This report provides a comprehensive analysis of the recombinant human Interleukin-11 (rHuIL-11) for injection market, encompassing market size estimations, growth forecasts, key drivers, and challenges. It delves into detailed segmentations by application, dosage form, and geographic region, offering a granular understanding of market dynamics. The report also profiles leading players, highlighting their market strategies and competitive landscape. Ultimately, this report serves as a valuable resource for stakeholders seeking to gain insights into this evolving market and make informed business decisions.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Xiamen Amoytop Biotech, SL Pharm, Qilu Pharmaceutical, Angde Biotech Pharmaceutical, Hangzhou Jiuyuan Gene Engineering.
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Recombinant Human Interleukin-11 for Injection," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Recombinant Human Interleukin-11 for Injection, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.