1. What is the projected Compound Annual Growth Rate (CAGR) of the Prefilled Epinephrine Auto-Injector?
The projected CAGR is approximately 8.5%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Prefilled Epinephrine Auto-Injector
Prefilled Epinephrine Auto-InjectorPrefilled Epinephrine Auto-Injector by Type (0.3 mg, 0.15 mg), by Application (Hospital, Clinic), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The global prefilled epinephrine auto-injector market is poised for significant expansion, propelled by the rising incidence of life-threatening allergic reactions (anaphylaxis) and amplified awareness of emergency response protocols. The market, valued at $3.3 billion in the base year 2025, is projected to achieve a Compound Annual Growth Rate (CAGR) of 8.5% from 2025 to 2033, reaching an estimated $3.3 billion by 2033. Key growth drivers include an aging demographic with increased allergy susceptibility, enhanced educational campaigns for the public and healthcare professionals emphasizing timely epinephrine administration, and continuous product innovation focused on improved auto-injector design and user experience. The market is segmented by dosage (0.15mg and 0.3mg) and application (hospital and clinic settings), with the 0.3mg dosage currently leading due to its suitability for adult and adolescent populations. While North America and Europe currently dominate due to robust healthcare spending and high anaphylaxis awareness, emerging markets in Asia Pacific are anticipated to experience substantial growth, driven by expanding healthcare infrastructure and rising disposable incomes.
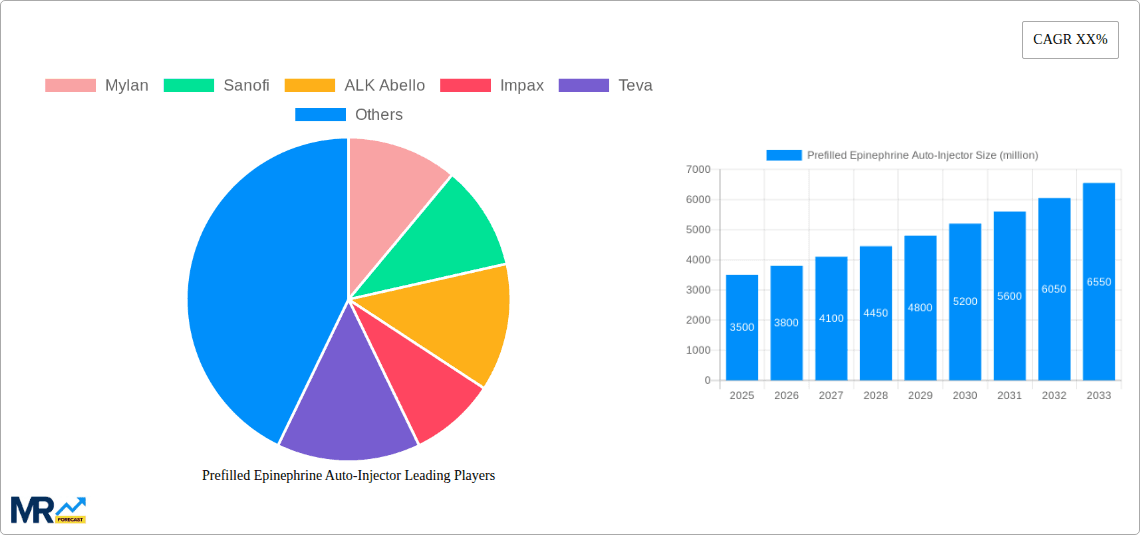

Market challenges include the high cost of products, potential adverse effects, and the persistent need for enhanced patient education and consistent auto-injector adherence. The competitive landscape is intense, with major pharmaceutical firms such as Mylan, Sanofi, and Teva alongside other key players like ALK Abello, Impax, and Amneal Pharmaceuticals. These companies are actively pursuing strategic initiatives including mergers, acquisitions, product line expansion, and global market penetration to fortify their competitive positions. Future market trajectories will be shaped by technological advancements in auto-injector design, such as dose counters and enhanced usability, alongside sustained public health campaigns to elevate awareness and mitigate anaphylaxis-related morbidity and mortality. The emergence of generic and biosimilar epinephrine auto-injectors will also play a pivotal role in market evolution.


The prefilled epinephrine auto-injector market is experiencing robust growth, driven by a confluence of factors. The rising prevalence of life-threatening allergic reactions, particularly anaphylaxis, is a primary catalyst. This increase is fueled by factors such as growing awareness of food allergies, heightened exposure to allergens in the environment, and an aging population with increased susceptibility to allergic conditions. The market's expansion is further propelled by continuous improvements in auto-injector design, leading to enhanced usability and patient compliance. These advancements include features such as simpler injection mechanisms, audible feedback, and improved training materials, all aimed at increasing ease of use during emergencies. Furthermore, stringent regulatory guidelines emphasizing the importance of readily available epinephrine for anaphylaxis management are driving market growth. Governments across numerous regions are promoting public awareness campaigns and advocating for wider accessibility of auto-injectors, leading to a surge in demand. The market is witnessing a shift towards innovative product offerings with improved features and greater patient convenience, contributing to the overall market expansion. The competitive landscape is also dynamic, with both established pharmaceutical companies and new entrants vying for market share, leading to product diversification and pricing strategies that influence market penetration and accessibility. Finally, the ongoing research and development efforts focusing on next-generation auto-injectors with features like dose counters and improved storage solutions further contribute to the market’s substantial expansion, projecting millions of units sold in the coming years. The overall trend indicates a consistently expanding market with substantial growth opportunities for industry players. The projected market size for 2025 is estimated at several million units, emphasizing the significant demand for this life-saving medication.
Several key factors are accelerating the growth of the prefilled epinephrine auto-injector market. Firstly, the escalating incidence of anaphylaxis, a severe and potentially fatal allergic reaction, demands readily available and easily administered treatment. This is driving a significant increase in the demand for auto-injectors. Secondly, heightened public awareness campaigns, educational initiatives, and improved physician training regarding the proper diagnosis and management of anaphylaxis are increasing the utilization of epinephrine auto-injectors. These educational efforts effectively communicate the critical role of prompt epinephrine administration in mitigating life-threatening consequences. Thirdly, continuous technological advancements in auto-injector design are enhancing usability and reducing the occurrence of injection errors. Features like simpler injection mechanisms, clear instructions, and audible feedback are making auto-injectors more accessible and user-friendly, particularly for individuals administering the injection in stressful emergency situations. Finally, favorable regulatory environments supporting the wider availability and accessibility of epinephrine auto-injectors are providing a crucial impetus for market growth. Regulations often mandate that schools, workplaces, and public venues maintain stocks of these devices, leading to increased demand. This combined effect of increased incidence, improved awareness, design improvements, and supportive regulations is fueling significant growth in this vital sector of the healthcare market.
Despite the significant growth potential, the prefilled epinephrine auto-injector market faces certain challenges. One major obstacle is the high cost of auto-injectors, creating financial barriers for some patients, particularly those lacking adequate insurance coverage. This cost constraint limits accessibility and hinders market penetration. Another challenge involves the relatively short shelf life of the medication, requiring frequent replacement and potentially leading to waste. This contributes to both the overall cost and logistical difficulties in managing inventory. Additionally, concerns regarding the proper storage and handling of the auto-injectors, particularly in extreme temperatures, pose a significant hurdle. Improper storage can lead to medication degradation, affecting efficacy and potentially jeopardizing patient safety. The complexity of administering the injection, even with improved designs, can also cause difficulties for some individuals, leading to suboptimal use or injection errors. Furthermore, the potential for side effects, albeit rare, can introduce hesitation among some patients, despite the life-saving potential of the treatment. Addressing these cost, storage, usability, and potential side effect concerns is critical to overcome these market restraints and ensure optimal patient care and market growth.
The 0.3 mg segment of the prefilled epinephrine auto-injector market is expected to dominate due to its suitability for a broader range of patients and its efficacy in treating severe anaphylactic reactions. This dosage is often the preferred choice for adults and adolescents, creating a larger market segment.
0.3 mg Segment Dominance: This segment will likely hold the largest market share throughout the forecast period (2025-2033), fueled by the higher incidence of anaphylaxis in adults and adolescents who generally require a higher dose of epinephrine.
Hospital Application Segment Growth: Hospitals and clinics will remain significant users of prefilled epinephrine auto-injectors, given their crucial role in managing emergency situations and providing immediate treatment to patients experiencing anaphylaxis. This segment's consistent demand contributes to considerable market revenue.
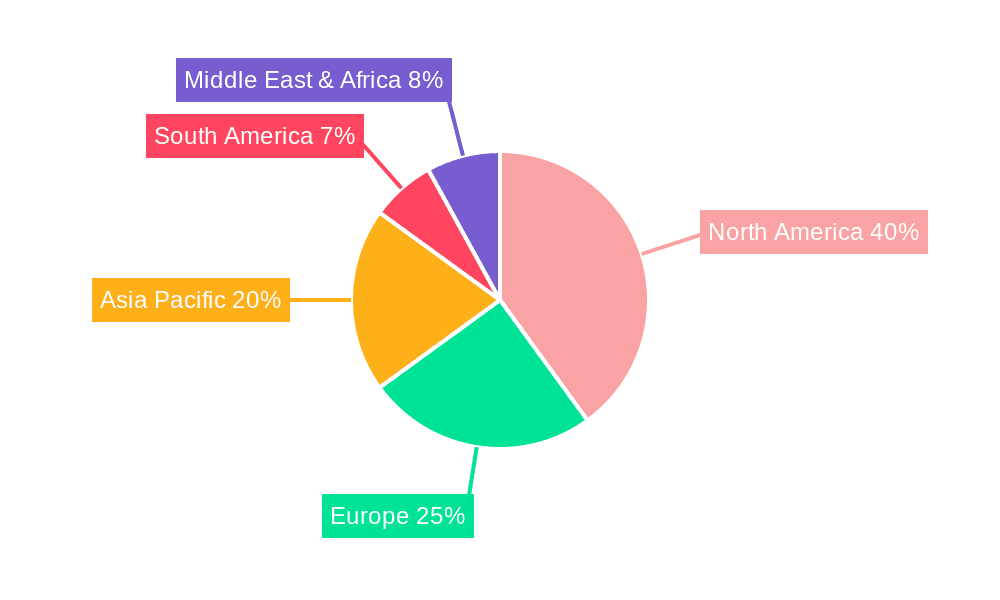
North America Market Leadership: North America is expected to maintain its leading position in the prefilled epinephrine auto-injector market, driven by factors such as high awareness of allergic diseases, robust healthcare infrastructure, and a relatively high prevalence of anaphylaxis.
Europe's Significant Contribution: Europe will also show substantial growth, propelled by increasing healthcare spending, rising awareness of allergic conditions, and improving healthcare infrastructure.
Emerging Markets' Potential: While currently smaller, emerging markets in Asia-Pacific, Latin America, and the Middle East and Africa exhibit promising growth potential due to increasing awareness of allergies and improved healthcare access, representing future opportunities.
In summary, the 0.3 mg segment and the hospital application sector are projected to lead market growth, with North America and Europe remaining major market players. However, emerging economies offer significant potential for future expansion. This diverse distribution underlines the widespread need for effective and readily available epinephrine auto-injectors across various demographics and geographic locations. The market size, measured in millions of units, significantly contributes to the overall healthcare landscape.
Several factors are catalyzing growth within the prefilled epinephrine auto-injector industry. These include the increasing prevalence of allergies, particularly anaphylaxis, leading to higher demand. Improved product designs with enhanced usability and patient-friendly features are boosting acceptance and use. Stronger regulatory support and public awareness campaigns are educating the public and healthcare professionals about the critical importance of prompt epinephrine administration. The ongoing development of innovative auto-injectors with improved features and enhanced safety further fuels market expansion.
This report provides a thorough analysis of the prefilled epinephrine auto-injector market, encompassing market trends, driving forces, challenges, key market segments, leading players, and significant developments. It offers valuable insights for stakeholders involved in the manufacturing, distribution, and use of these life-saving devices, projecting significant growth and substantial market size in millions of units within the forecast period.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 8.5% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 8.5%.
Key companies in the market include Mylan, Sanofi, ALK Abello, Impax, Teva, Amneal Pharmaceuticals LLC, .
The market segments include Type, Application.
The market size is estimated to be USD 3.3 billion as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in billion and volume, measured in K.
Yes, the market keyword associated with the report is "Prefilled Epinephrine Auto-Injector," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Prefilled Epinephrine Auto-Injector, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.