1. What is the projected Compound Annual Growth Rate (CAGR) of the Pharmaceutical Track And Trace System?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Pharmaceutical Track And Trace System
Pharmaceutical Track And Trace SystemPharmaceutical Track And Trace System by Type (/> Barcodes Pharmaceutical Track And Trace System, Real-time Locating System (RTLS) Pharmaceutical Track And Trace System, Radio-frequency Identification (RFID) Tags Pharmaceutical Track And Trace System), by Application (/> Hospital Pharmacy, Retail Pharmacy, Online Pharmacy), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The pharmaceutical track and trace system market is experiencing robust growth, driven by increasing regulatory pressures for drug counterfeiting prevention and enhanced supply chain visibility. The market, valued at approximately $2 billion in 2025, is projected to exhibit a Compound Annual Growth Rate (CAGR) of 15% from 2025 to 2033, reaching an estimated $6 billion by 2033. This expansion is fueled by several key factors. Firstly, the rising prevalence of counterfeit drugs poses a significant threat to public health and necessitates stringent tracking mechanisms. Secondly, advancements in serialization technologies, including RFID and barcode systems, are improving the efficiency and accuracy of track and trace solutions. Thirdly, growing adoption of cloud-based platforms offers better data management and analytics capabilities, further driving market growth. Leading players like Marchesini Group, Weber Marking Systems, and others are actively investing in R&D to develop innovative solutions that meet evolving regulatory requirements and enhance supply chain security.
Market segmentation reveals significant opportunities across various regions. North America currently holds a dominant market share, attributable to stringent regulatory frameworks and high pharmaceutical industry spending. However, the Asia-Pacific region is expected to witness the fastest growth rate due to rising pharmaceutical consumption and supportive government initiatives to combat drug counterfeiting. Despite the positive outlook, challenges remain. High implementation costs and integration complexities associated with track and trace systems may hinder adoption, especially among smaller pharmaceutical companies. Furthermore, maintaining data security and ensuring compliance with varying international regulations present ongoing hurdles for market participants. The overall trajectory, however, indicates a significant and sustained period of growth, bolstered by both technological advancements and regulatory mandates.
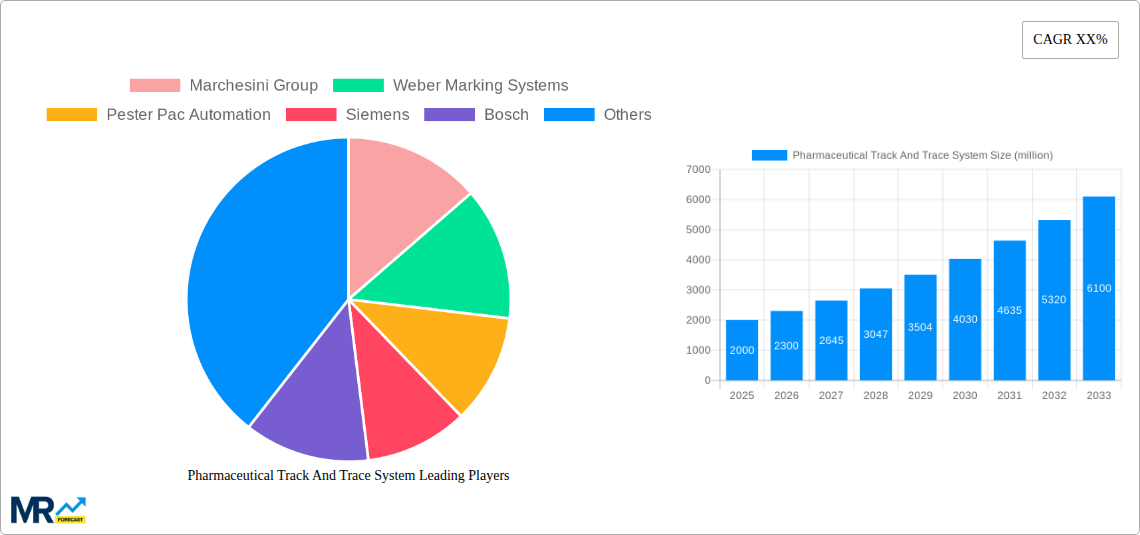
The pharmaceutical track and trace system market is experiencing explosive growth, projected to reach multi-billion dollar valuations by 2033. Driven by stringent regulatory compliance mandates globally and a rising consumer demand for product authenticity and safety, the market shows a consistent upward trajectory. The historical period (2019-2024) witnessed significant adoption, particularly in developed regions, with the base year 2025 marking a crucial inflection point. The estimated market value for 2025 is already in the several hundred million unit range, poised for substantial expansion during the forecast period (2025-2033). This growth is fueled by technological advancements, including the integration of AI and machine learning for improved data analysis and predictive capabilities. The market is witnessing a shift towards cloud-based solutions, offering enhanced scalability and accessibility. Furthermore, the increasing prevalence of counterfeit drugs and the need for robust supply chain security are significant drivers. The market is segmented by technology (RFID, barcode, etc.), by application (serialization, aggregation, etc.), and by end-user (pharmaceutical manufacturers, distributors, etc.), with each segment contributing uniquely to the overall growth. The competitive landscape is dynamic, with both established players and emerging technology providers vying for market share. Significant mergers and acquisitions are anticipated, leading to further consolidation in the coming years. The market is also seeing increasing focus on interoperability and data standardization to improve overall efficiency and traceability across the entire pharmaceutical supply chain. The trend towards digitalization and the increasing adoption of advanced analytics are expected to further enhance the efficiency and effectiveness of track and trace systems, contributing significantly to the market's future growth potential.
Several key factors are propelling the growth of the pharmaceutical track and trace system market. Stringent regulatory frameworks, such as the Drug Supply Chain Security Act (DSCSA) in the US and similar regulations worldwide, mandate the implementation of robust track and trace solutions. This regulatory pressure is the primary driver, compelling pharmaceutical companies of all sizes to invest in these systems to ensure compliance and avoid hefty penalties. Furthermore, the escalating problem of counterfeit drugs poses a significant threat to public health and safety. Track and trace systems provide a crucial defense mechanism against this threat by enabling the verification of product authenticity throughout the supply chain. Consumer demand for transparency and product safety is also on the rise, driving the adoption of these systems. Consumers are increasingly demanding verifiable proof of product authenticity and are more likely to trust brands that utilize track and trace technology. The continuous technological advancements in areas such as RFID, blockchain, and AI are further enhancing the capabilities of track and trace systems, making them more efficient, secure, and cost-effective. This technological evolution is also attracting new entrants to the market, fostering competition and driving innovation. Finally, the increasing need for efficient supply chain management and improved inventory control are also contributing to the market's growth, as track and trace systems provide valuable real-time visibility into product movement and location.
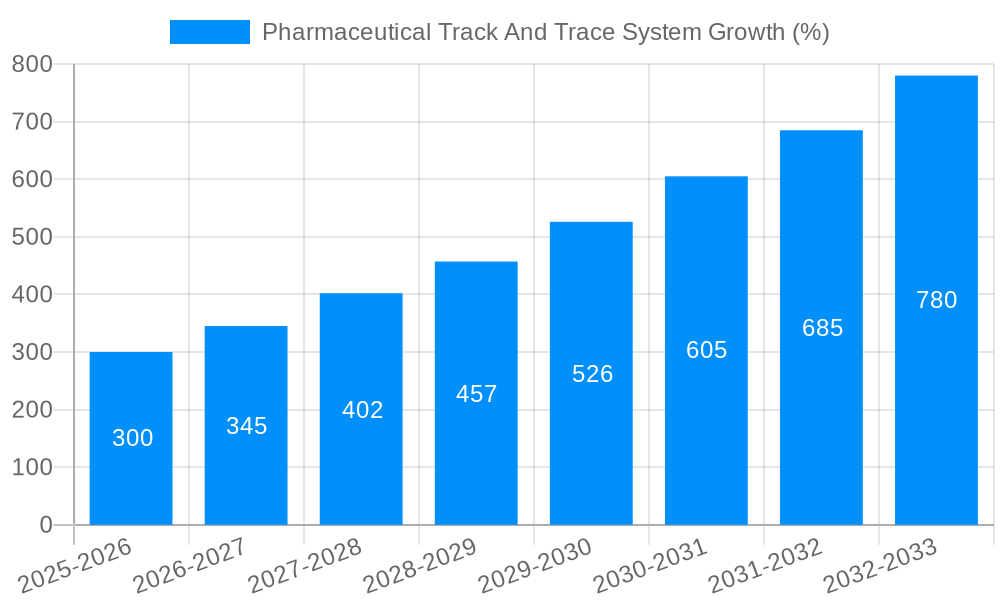
Despite the significant growth potential, the pharmaceutical track and trace system market faces several challenges and restraints. The high initial investment costs associated with implementing these systems can be a significant barrier, particularly for smaller pharmaceutical companies. The integration of these systems with existing IT infrastructure can also be complex and time-consuming, requiring substantial resources and expertise. Data security and privacy concerns are also crucial. These systems handle sensitive patient and product data, making data breaches a major risk. Ensuring robust data security measures is paramount to maintaining consumer trust and regulatory compliance. Interoperability between different track and trace systems remains a challenge. The lack of standardization across different systems can create difficulties in data exchange and information sharing across the entire supply chain. The complexity of managing large volumes of data generated by these systems also poses a challenge. Effective data analytics capabilities are essential to derive meaningful insights from the vast amount of data generated. Finally, the ongoing evolution of technology necessitates continuous upgrades and maintenance, adding to the overall cost of ownership. Addressing these challenges requires collaboration between stakeholders, including regulatory bodies, technology providers, and pharmaceutical companies, to promote standardization, interoperability, and cost-effective solutions.
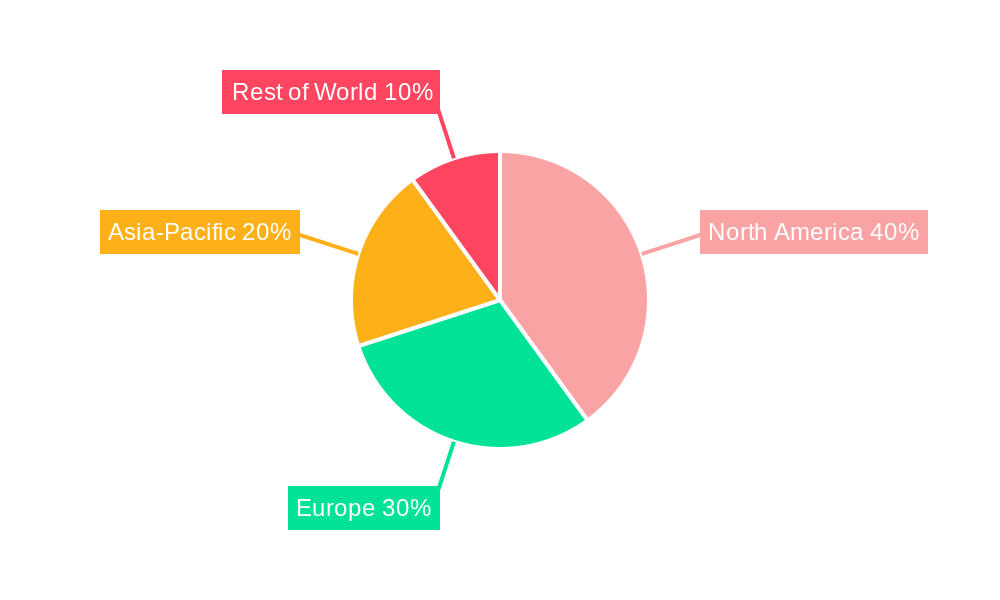
North America: The region is expected to dominate the market due to stringent regulations like the DSCSA, high adoption rates, and advanced technological infrastructure. The high prevalence of counterfeit drugs further fuels demand in this region. The market value here is projected to be in the hundreds of millions of units by 2033.
Europe: Strong regulatory frameworks and a well-established pharmaceutical industry contribute to significant market growth. The focus on patient safety and product authenticity drives adoption rates. The market value is likely to be comparable to North America in scale.
Asia-Pacific: This region is experiencing rapid growth, driven by increasing pharmaceutical production and consumption. However, challenges related to infrastructure and regulatory harmonization might slightly slow down the adoption rate compared to North America and Europe. Still, the market is expected to see substantial growth in the coming years, reaching values in the hundreds of millions of units.
Serialization Segment: This segment is projected to hold a substantial market share due to its mandatory implementation in several regions for ensuring product authenticity and traceability at the individual unit level. This segment's value is predicted to be one of the highest among all segments.
Aggregation Segment: This segment focuses on combining serialized units into larger packages, providing a higher level of traceability and facilitating efficient supply chain management. Its value is expected to closely follow the serialization segment due to its intrinsic linkage.
Pharmaceutical Manufacturers: This segment will be the key driver, as manufacturers are the primary adopters of track and trace systems to meet regulatory requirements and protect their brand reputation.
The paragraph above provides context by clarifying that regional dominance is primarily influenced by regulatory stringency and market maturity. Meanwhile, the dominance of specific segments is linked to regulatory mandates (serialization) and efficient supply chain management (aggregation). The market value figures, projected to be in the hundreds of millions of units across regions, clearly indicate a significant and expanding market. The dominance of pharmaceutical manufacturers as the main users of these systems also reinforces the fundamental role of these systems within the pharmaceutical industry.
Several factors are catalyzing growth in the pharmaceutical track and trace system industry. The increasing adoption of advanced technologies like blockchain, AI, and IoT is improving the efficiency, security, and scalability of track and trace solutions. Furthermore, the rising prevalence of counterfeit pharmaceuticals necessitates robust track and trace solutions to safeguard public health. Simultaneously, the growing emphasis on supply chain transparency and patient safety is pushing companies to adopt these systems. This combination of technological advancements, regulatory pressure, and consumer demand creates a fertile ground for rapid market expansion.
This report provides a comprehensive overview of the pharmaceutical track and trace system market, offering valuable insights into market trends, driving forces, challenges, and growth opportunities. It covers key players, technological advancements, and regulatory developments, providing a detailed analysis of the market's past, present, and future. The report is an essential resource for industry stakeholders seeking a thorough understanding of this dynamic and rapidly evolving market.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Marchesini Group, Weber Marking Systems, Pester Pac Automation, Siemens, Bosch, Körber, Uhlmann Packaging System.
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Pharmaceutical Track And Trace System," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Pharmaceutical Track And Trace System, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.