1. What is the projected Compound Annual Growth Rate (CAGR) of the Pharmaceutical Prepacked Chromatography Columns?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Pharmaceutical Prepacked Chromatography Columns
Pharmaceutical Prepacked Chromatography ColumnsPharmaceutical Prepacked Chromatography Columns by Application (Process Validation, Commercial and GMP Manufacturing, World Pharmaceutical Prepacked Chromatography Columns Production ), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
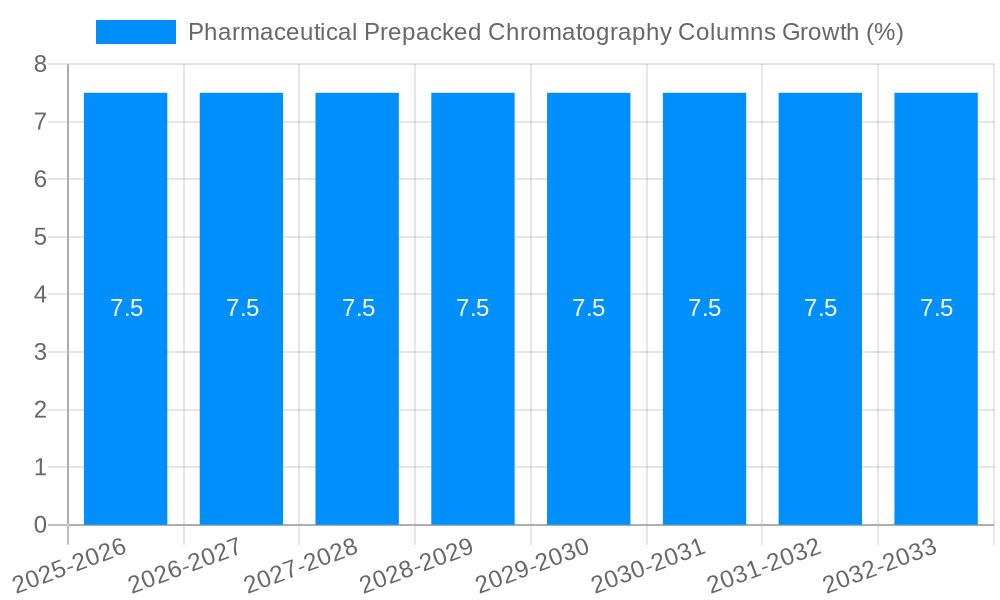
The global Pharmaceutical Prepacked Chromatography Columns market is poised for significant expansion, projected to reach a substantial USD 1866.3 million by 2025. This growth is fueled by the increasing complexity of pharmaceutical development and manufacturing, necessitating advanced separation techniques like chromatography. The rising demand for biologics and biopharmaceuticals, which often require highly specific purification processes, is a primary catalyst. Furthermore, stringent regulatory requirements for drug purity and safety are driving the adoption of pre-validated and ready-to-use chromatography columns, reducing validation timelines and costs for pharmaceutical companies. The trend towards in-house manufacturing capabilities and the growing pipeline of new drug candidates, particularly in oncology, autoimmune diseases, and infectious diseases, further underscore the market's upward trajectory. The market is expected to experience a Compound Annual Growth Rate (CAGR) of approximately 7.5% over the forecast period of 2025-2033, reflecting sustained demand and innovation in this critical segment of biopharmaceutical production.
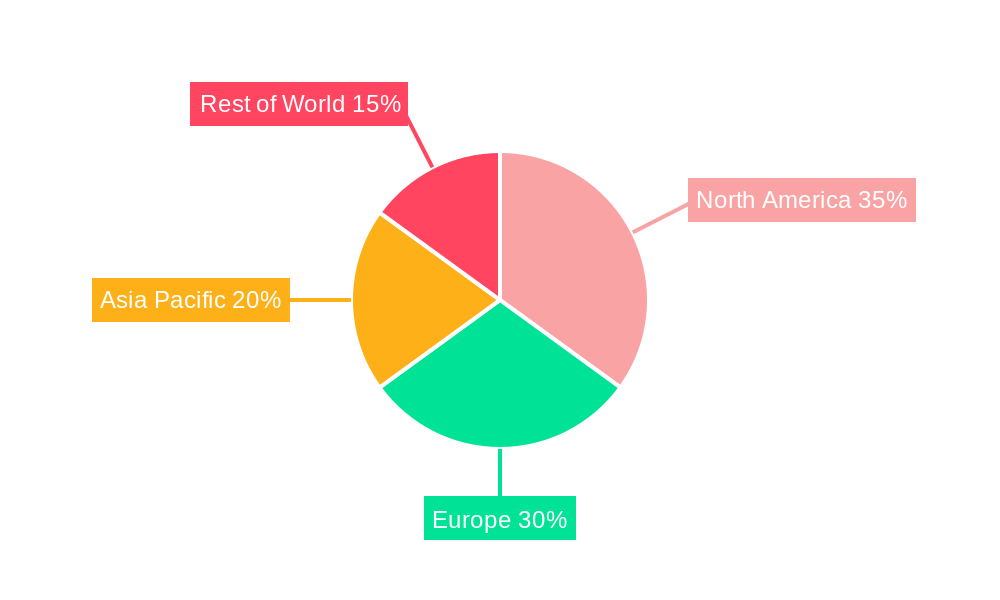
Key applications driving this market include process validation and commercial and GMP manufacturing. Process validation relies heavily on the reproducible and efficient separation capabilities offered by prepacked columns, ensuring the quality and consistency of pharmaceutical products. For commercial and GMP manufacturing, prepacked columns offer a significant advantage in terms of speed, reliability, and reduced risk of contamination, crucial for large-scale production. Major players such as Agilent Technologies, Waters Corporation, Thermo Fisher Scientific, and Merck are at the forefront of this market, continually investing in research and development to offer innovative solutions. The market is global in scope, with North America and Europe currently leading in terms of market share due to established biopharmaceutical industries and robust R&D activities. However, the Asia Pacific region is anticipated to witness the fastest growth, driven by increasing pharmaceutical investments, expanding manufacturing capacities, and a growing focus on biopharmaceutical production in countries like China and India.
This report provides a comprehensive analysis of the global Pharmaceutical Prepacked Chromatography Columns market, offering insights into its current landscape, future trajectory, and key influencing factors. The study encompasses a detailed examination of market trends, driving forces, challenges, regional dynamics, and the competitive landscape from the historical period of 2019-2024, through the base year of 2025, and extending into the forecast period of 2025-2033.
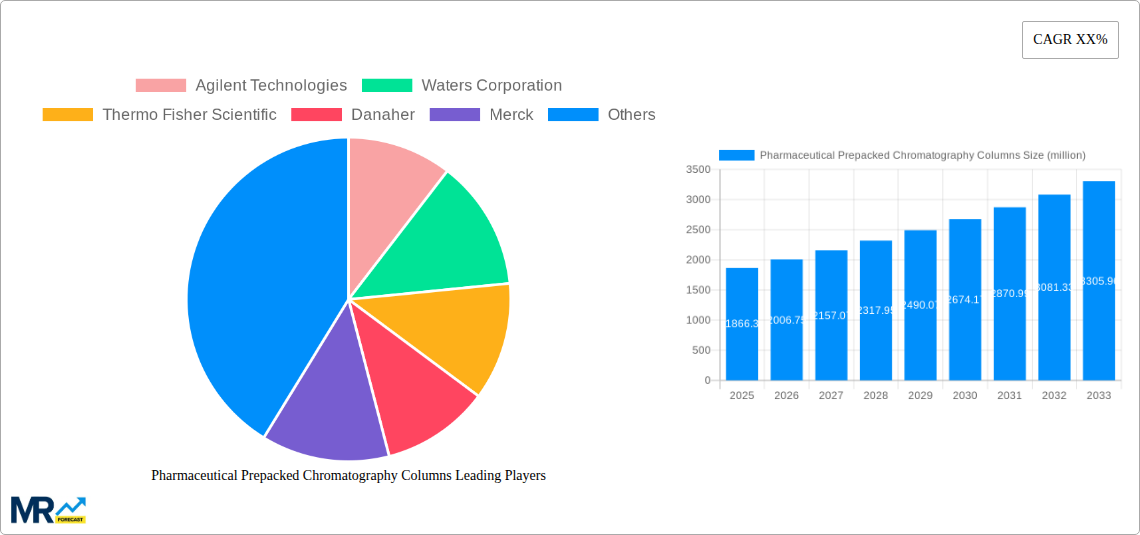
The pharmaceutical prepacked chromatography columns market is experiencing a robust expansion, driven by a confluence of technological advancements, evolving regulatory landscapes, and the accelerating pace of drug discovery and development. XXX, a key indicator of market health, reveals a consistent upward trajectory in demand for these specialized chromatography solutions. The inherent advantages of prepacked columns, such as their plug-and-play nature, reduced manual labor, enhanced reproducibility, and minimized risk of contamination, are increasingly being recognized and adopted by pharmaceutical manufacturers and contract research organizations (CROs). This shift towards greater efficiency and reliability in purification processes is a fundamental trend. Furthermore, the growing complexity of biologic drugs, including monoclonal antibodies, recombinant proteins, and gene therapies, necessitates highly efficient and scalable purification methods. Prepacked columns are at the forefront of addressing these purification challenges, offering tailored solutions for various therapeutic modalities. The increasing focus on process analytical technology (PAT) and real-time monitoring also favors prepacked columns, as they are amenable to integration with advanced analytical tools for better process control and optimization. The market is also witnessing a significant trend towards the development of novel stationary phases with improved selectivity and binding capacities, catering to the purification of increasingly challenging biomolecules. The global production of pharmaceutical prepacked chromatography columns is projected to reach a substantial figure in the millions of units annually, reflecting the immense scale of the pharmaceutical industry’s reliance on these critical purification tools. The market's growth is further fueled by the expanding biologics pipeline, the rise of personalized medicine, and the increasing outsourcing of drug manufacturing activities, all of which contribute to a sustained demand for high-quality, ready-to-use chromatography solutions.
Several key factors are acting as powerful engines driving the growth of the pharmaceutical prepacked chromatography columns market. Foremost among these is the burgeoning biologics market. As the development and commercialization of complex protein-based therapeutics, monoclonal antibodies, and gene therapies continue to surge, the demand for efficient and reproducible purification techniques escalates. Prepacked chromatography columns offer a significant advantage in handling these intricate molecules, providing consistent results and reducing the risk of product degradation or contamination that can be associated with manual packing. Furthermore, the intensifying regulatory scrutiny and the global push towards Good Manufacturing Practices (GMP) are compelling pharmaceutical companies to adopt robust and validated purification processes. Prepacked columns, with their inherent standardization and traceability, align perfectly with these stringent regulatory requirements, offering a lower risk profile and facilitating faster regulatory approvals. The increasing adoption of automation and high-throughput screening in drug discovery and development also plays a crucial role. Prepacked columns seamlessly integrate into automated workflows, enabling researchers to purify larger numbers of samples with greater speed and accuracy, thereby accelerating the identification of promising drug candidates. The growing trend of outsourcing drug manufacturing to Contract Manufacturing Organizations (CMOs) and Contract Development and Manufacturing Organizations (CDMOs) further amplifies the demand for prepacked columns, as these organizations strive for operational efficiency and cost-effectiveness.
Despite the promising growth trajectory, the pharmaceutical prepacked chromatography columns market is not without its hurdles. A primary challenge revolves around the initial cost of prepacked columns compared to traditional unpackaged resins. While the long-term benefits in terms of efficiency and reduced labor costs are evident, the upfront investment can be a deterrent, particularly for smaller research labs or organizations with budget constraints. The issue of resin and column compatibility also presents a restraint. While manufacturers offer a wide range of prepacked options, ensuring optimal compatibility between the specific biomolecule being purified, the chosen stationary phase, and the buffer conditions can require extensive testing and validation, adding to the overall development timeline and cost. Furthermore, the limited shelf-life of some prepacked columns, especially those containing sensitive biological materials or requiring specific storage conditions, can lead to inventory management challenges and potential product wastage. The development of specialized prepacked columns for highly niche applications or for the purification of emerging therapeutic modalities can also be complex and time-consuming, potentially slowing down market penetration in these specific areas. Finally, the need for specialized training to effectively operate and troubleshoot advanced prepacked chromatography systems, although less so than with manual packing, can still pose a minor barrier to widespread adoption in certain settings.
The Commercial and GMP Manufacturing segment is poised to dominate the pharmaceutical prepacked chromatography columns market, driven by the sheer scale and critical importance of large-scale drug production. This segment is characterized by high-volume purification of active pharmaceutical ingredients (APIs) and biologics, where reproducibility, scalability, and regulatory compliance are paramount. Prepacked columns are instrumental in ensuring lot-to-lot consistency and minimizing the risk of costly batch failures during commercial manufacturing, making them indispensable tools for pharmaceutical giants and CMOs alike. The increasing global demand for essential medicines, vaccines, and complex biologics directly translates into a higher consumption of prepacked chromatography columns for their large-scale purification.
Within this segment, the North America region, encompassing the United States and Canada, is expected to emerge as a leading market. Several factors contribute to this dominance:
The Commercial and GMP Manufacturing segment, particularly within the North America region, will therefore represent the largest share of the global pharmaceutical prepacked chromatography columns market throughout the forecast period. The ability of prepacked columns to streamline the purification process, ensure product quality, and meet stringent regulatory requirements makes them the preferred choice for commercial-scale production.
The pharmaceutical prepacked chromatography columns industry is experiencing significant growth catalysts, primarily driven by the burgeoning biologics sector and the increasing complexity of drug molecules. The demand for purified biologics, such as monoclonal antibodies and gene therapies, is soaring, and prepacked columns offer a highly efficient and reproducible solution for their isolation. Furthermore, the global emphasis on faster drug development timelines and the implementation of stringent regulatory standards are pushing manufacturers towards adopting standardized and validated purification methods, which prepacked columns readily provide.
This comprehensive report delves into the intricate dynamics of the pharmaceutical prepacked chromatography columns market, providing a holistic view of its present and future landscape. It meticulously analyzes the market size, segmentation, and regional distribution, offering valuable quantitative data and qualitative insights. The report further scrutinizes the key trends shaping the industry, such as the increasing demand for high-performance resins and automated purification systems. It also explores the driving forces, including the expansion of the biologics market and the tightening regulatory environment, while acknowledging the challenges and restraints that the market faces, such as cost considerations and the need for specialized expertise.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Agilent Technologies, Waters Corporation, Thermo Fisher Scientific, Danaher, Merck, Bio-Rad, Sartorius, Repligen Corporation, Tosoh Bioscience, Astrea Bioseparations, Showa Denko, .
The market segments include Application.
The market size is estimated to be USD 1866.3 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Pharmaceutical Prepacked Chromatography Columns," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Pharmaceutical Prepacked Chromatography Columns, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.