1. What is the projected Compound Annual Growth Rate (CAGR) of the Pharmaceutical Asset Management?
The projected CAGR is approximately 4.5%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Pharmaceutical Asset Management
Pharmaceutical Asset ManagementPharmaceutical Asset Management by Type (Hardware(Pharma Asset), Software(Pharma Asset)), by Application (Anti-drug Counterfeiting, Supply Chain Management), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The global Pharmaceutical Asset Management market is poised for robust expansion, projected to reach approximately USD 112.2 million by 2025, with an anticipated Compound Annual Growth Rate (CAGR) of 4.5% throughout the forecast period of 2025-2033. This growth trajectory is underpinned by several critical drivers, most notably the escalating need to combat drug counterfeiting. The increasing sophistication of counterfeit operations poses a significant threat to patient safety and brand integrity, compelling pharmaceutical companies to invest heavily in advanced asset management solutions. Furthermore, the drive for enhanced supply chain efficiency and transparency is a major catalyst. With complex global pharmaceutical supply chains, ensuring the integrity, traceability, and optimal utilization of assets – from raw materials to finished products – is paramount. Technologies that facilitate real-time tracking, inventory management, and regulatory compliance are therefore in high demand.
The market is segmented into Hardware, encompassing solutions for physical pharmaceutical assets, and Software, which includes digital management platforms. Applications predominantly focus on combating anti-drug counterfeiting and optimizing supply chain management. Key players such as McKesson Corporation, Cerner Corporation, and Siemens Healthcare are at the forefront, driving innovation and market penetration. Geographically, North America, particularly the United States, is expected to lead the market due to its advanced healthcare infrastructure and stringent regulatory environment. Asia Pacific, with its burgeoning pharmaceutical industry and increasing focus on drug safety, is also anticipated to exhibit significant growth. While the market is primed for expansion, certain restraints, such as the high initial investment cost for implementing sophisticated asset management systems and the need for extensive training, may temper growth in some regions. However, the overwhelming demand for secure and efficient pharmaceutical operations is expected to outweigh these challenges.
This report offers a comprehensive analysis of the Pharmaceutical Asset Management market, providing in-depth insights into its current trajectory, future potential, and the intricate ecosystem that defines it. With a study period spanning from 2019 to 2033, a base year of 2025, and a forecast period of 2025-2033, this research delves into the historical performance (2019-2024) and projects the significant transformations expected in the coming decade. Our analysis quantifies key market movements, including asset values in the millions of units, and dissects the market across crucial segments like Hardware (Pharma Asset) and Software (Pharma Asset), as well as vital applications such as Anti-drug Counterfeiting and Supply Chain Management. We also explore the pivotal industry developments shaping this dynamic landscape.
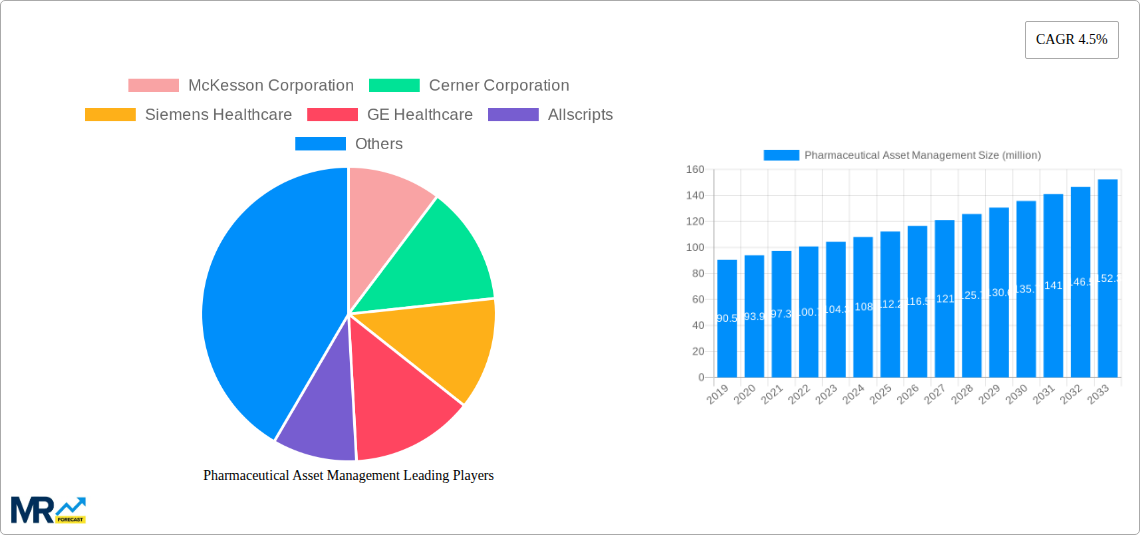
XXX paints a compelling picture of the Pharmaceutical Asset Management market, characterized by a steady and strategic evolution driven by an increasing demand for enhanced efficiency, robust security, and stringent regulatory compliance. The market, valued in the millions of units, is witnessing a significant shift from fragmented, manual asset tracking to sophisticated, integrated digital solutions. A paramount trend is the burgeoning adoption of IoT-enabled devices for real-time monitoring of pharmaceutical assets, encompassing everything from temperature-sensitive biologics to high-value finished goods. This granular visibility allows for proactive intervention in case of deviations, minimizing spoilage and ensuring product integrity throughout the supply chain. Furthermore, the integration of advanced analytics and artificial intelligence (AI) is revolutionizing how pharmaceutical companies manage their assets. AI-powered algorithms are now being employed for predictive maintenance of manufacturing equipment, optimizing inventory levels to reduce waste and stockouts, and even forecasting demand with greater accuracy.
The increasing sophistication of counterfeiting operations globally has propelled Anti-drug Counterfeiting applications to the forefront. Companies are investing heavily in technologies that provide unique identifiers, secure serialization, and blockchain-based tracking systems to verify the authenticity of pharmaceuticals from manufacturing to the end consumer. This not only protects brand reputation but, more importantly, safeguards patient safety. Simultaneously, Supply Chain Management applications are undergoing a radical transformation. Leveraging digital twins and sophisticated control towers, organizations are gaining unprecedented transparency into their entire supply chain, enabling them to identify bottlenecks, optimize logistics, and respond rapidly to disruptions. The move towards cloud-based solutions is also a significant trend, offering scalability, accessibility, and cost-effectiveness for pharmaceutical companies of all sizes. As the industry navigates the complexities of a globalized market and an ever-increasing regulatory burden, the demand for intelligent, data-driven pharmaceutical asset management solutions is set to accelerate, moving beyond simple tracking to encompass intelligent control and predictive optimization. The market is poised for significant growth, fueled by innovation and the relentless pursuit of operational excellence.
The pharmaceutical asset management market is experiencing robust growth, propelled by a confluence of critical factors that underscore the industry's imperative to optimize operations and ensure product integrity. Foremost among these is the escalating complexity of global pharmaceutical supply chains. As companies expand their reach and product portfolios, the need for precise tracking, inventory control, and real-time visibility across a vast network of manufacturing facilities, distribution centers, and end-point delivery locations becomes paramount. This complexity directly translates into a demand for sophisticated asset management solutions that can handle immense volumes and intricate logistical pathways, measured in the millions of units.
Furthermore, the ever-tightening regulatory landscape worldwide plays a crucial role. Stringent requirements for serialization, track-and-trace capabilities, and Good Distribution Practices (GDP) necessitate advanced systems to ensure compliance and avoid costly penalties. Pharmaceutical companies are actively seeking solutions that not only meet these mandates but also provide an audit trail for every asset. The persistent and growing threat of counterfeit drugs is another significant driver. The immense financial and reputational damage, coupled with the profound risk to patient safety, compels manufacturers to invest in robust anti-counterfeiting measures. This includes technologies for product authentication, secure labeling, and blockchain integration to create an immutable record of an asset's journey. The pursuit of operational efficiency and cost reduction also fuels market growth. By accurately managing assets, companies can minimize waste due to spoilage, reduce the costs associated with excess inventory, and optimize resource allocation, all of which contribute to a healthier bottom line.
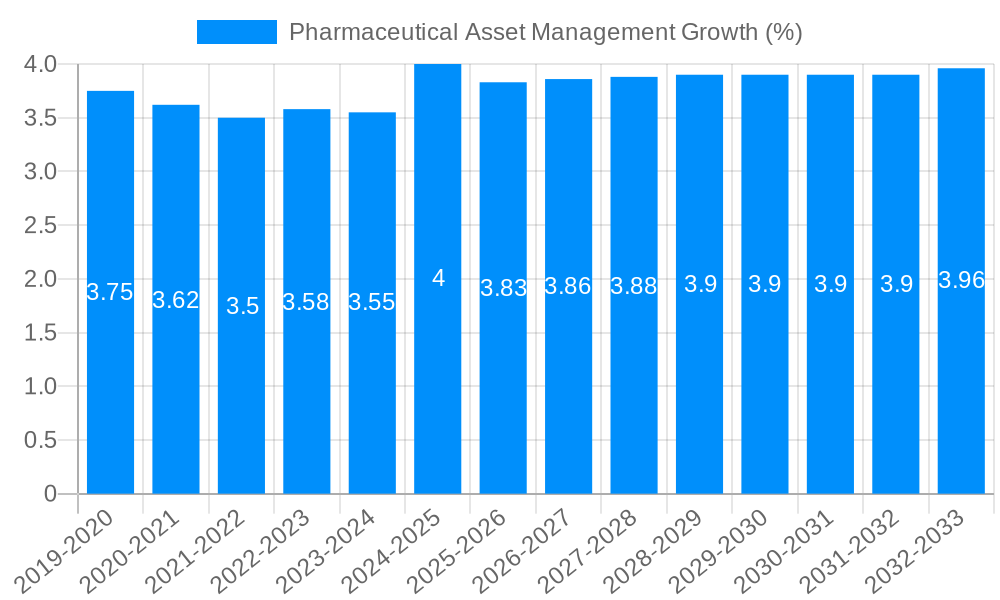
Despite the significant growth and inherent value proposition of pharmaceutical asset management solutions, several challenges and restraints temper the market’s full potential. A primary hurdle is the substantial upfront investment required for implementing advanced asset management systems, particularly for smaller and medium-sized enterprises (SMEs) within the pharmaceutical sector. The integration of new software, hardware, and potentially the upgrade of existing infrastructure can represent a considerable capital expenditure, leading to a cautious approach by some organizations. Furthermore, the complexity of integrating these new systems with legacy IT infrastructure and existing enterprise resource planning (ERP) systems can be a significant technical challenge. Data silos and a lack of interoperability between different platforms can hinder the seamless flow of information, thereby limiting the overall effectiveness of asset management strategies, even with assets valued in the millions of units.
The scarcity of skilled personnel capable of managing and optimizing these sophisticated digital solutions also presents a restraint. Implementing and maintaining advanced asset management tools requires specialized expertise in areas such as data analytics, IoT management, and cybersecurity, which can be difficult to find and retain. Cybersecurity threats are another growing concern. As pharmaceutical companies digitize their asset management processes, they become more vulnerable to cyberattacks that could compromise sensitive data, disrupt operations, or even lead to product diversion. Ensuring the security of the entire asset lifecycle, from manufacturing to distribution, is a continuous and resource-intensive undertaking. Finally, resistance to change within organizations can also act as a restraint. Employees accustomed to traditional methods may be reluctant to adopt new technologies and processes, necessitating comprehensive training and change management initiatives to ensure successful adoption and widespread utilization of asset management systems.
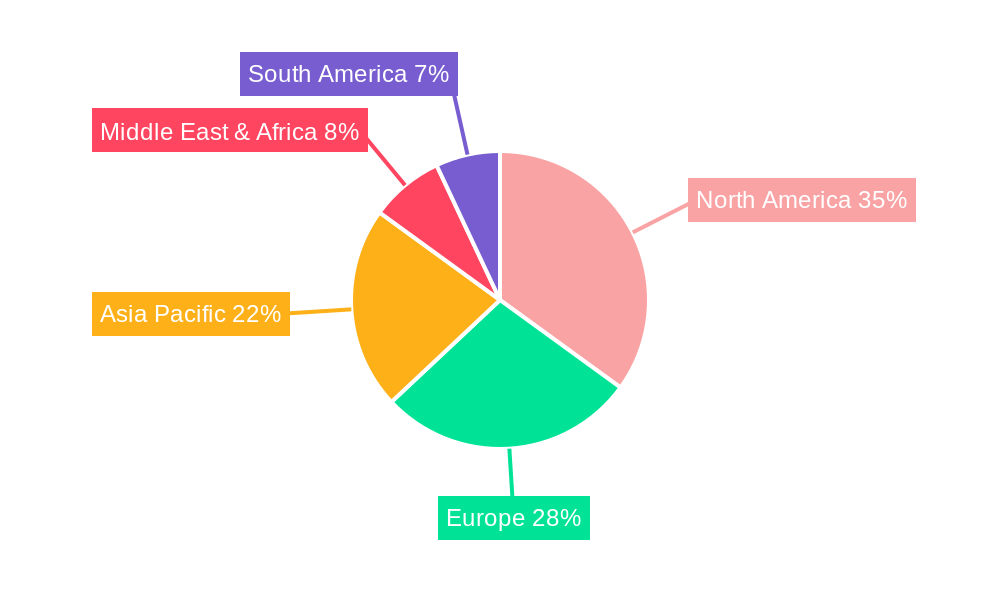
The Pharmaceutical Asset Management market is poised for significant growth and dominance by specific regions and segments, driven by a combination of factors including market maturity, regulatory stringency, and technological adoption rates. North America, particularly the United States, is expected to continue its leading position due to the presence of a large pharmaceutical manufacturing base, stringent regulatory requirements from the FDA, and a high adoption rate of advanced technologies. The emphasis on drug safety, supply chain integrity, and the proactive measures against counterfeit drugs are strong drivers in this region. The sheer volume of pharmaceutical products manufactured and distributed, often valued in the millions of units, necessitates robust asset management solutions.
In terms of segments, Software (Pharma Asset) is projected to dominate the market. This dominance stems from the increasing reliance on digital solutions for data management, analytics, and real-time tracking. Cloud-based software solutions are particularly gaining traction due to their scalability, accessibility, and cost-effectiveness. These software platforms enable comprehensive supply chain visibility, real-time monitoring of asset conditions (temperature, humidity), and advanced analytics for demand forecasting and inventory optimization.
Supply Chain Management as an application area is also a significant contributor to market dominance. The pharmaceutical industry’s inherent need for a secure, efficient, and compliant supply chain makes this application segment critical. Technologies enabling serialization, track-and-trace, and the prevention of diversion are paramount. The ability to monitor the entire journey of a drug, from raw material sourcing to patient delivery, is essential for ensuring product quality, patient safety, and regulatory adherence. The integration of blockchain technology for enhanced supply chain transparency and security further bolsters this segment.
Another key region exhibiting substantial growth potential is Europe. Countries like Germany, the United Kingdom, and Switzerland have well-established pharmaceutical industries, coupled with robust regulatory frameworks such as the EU's Falsified Medicines Directive (FMD). These regulations mandate serialized packaging and track-and-trace capabilities, driving the adoption of pharmaceutical asset management solutions. The growing focus on cold chain logistics for biologics and vaccines further propels the demand for advanced asset management systems in this region.
The Asia Pacific region, led by countries such as China and India, presents a rapidly expanding market. The increasing pharmaceutical manufacturing capacity, a growing domestic market, and a rising awareness of the need for quality and safety are key drivers. While regulatory frameworks are still evolving in some parts of the region, the trend towards stricter compliance and the adoption of global best practices is evident, leading to increased investments in asset management technologies. The sheer potential for growth in terms of production volume, with assets frequently reaching millions of units, positions Asia Pacific as a critical market for future expansion.
Within the Type segment, Hardware (Pharma Asset), including sensors, RFID tags, and tracking devices, plays a crucial supportive role to the software dominance. These hardware components are essential for capturing the real-time data that fuels the software solutions, enabling accurate tracking and monitoring. However, the overarching intelligence, analytics, and control reside within the software platforms, making them the primary driver of market value.
Several key catalysts are fueling the growth of the Pharmaceutical Asset Management industry. The escalating global demand for pharmaceuticals, driven by aging populations and rising healthcare access, necessitates more efficient and scalable asset management to ensure product availability. Furthermore, increasing governmental regulations worldwide mandating serialization, track-and-trace capabilities, and stringent quality control are compelling pharmaceutical companies to invest in advanced management systems to ensure compliance and avoid hefty penalties. The persistent threat of drug counterfeiting also acts as a significant catalyst, driving the adoption of technologies that enhance product authenticity and supply chain security. Finally, the continuous innovation in technologies like IoT, AI, and blockchain is enabling the development of more sophisticated, cost-effective, and integrated asset management solutions.
The pharmaceutical asset management landscape is shaped by a diverse array of established technology providers and specialized solution developers. Key players contributing to the market's evolution include:
The Pharmaceutical Asset Management sector has witnessed several key developments that have reshaped its trajectory and capabilities:
This report offers unparalleled comprehensive coverage of the Pharmaceutical Asset Management market, providing a holistic view of its dynamics and future potential. Our analysis meticulously dissects the market value, expressed in the millions of units, and delves deep into the interplay between hardware and software components, recognizing their symbiotic relationship in achieving optimal asset management. We explore the critical applications of Anti-drug Counterfeiting and Supply Chain Management, highlighting how these functionalities are becoming indispensable for pharmaceutical enterprises navigating a complex global landscape. Our research encompasses a detailed historical analysis from 2019 to 2024, providing a solid foundation for understanding past trends and market evolution. The base year of 2025 serves as a critical juncture for evaluating current market conditions, while the forecast period from 2025 to 2033 offers strategic projections and identifies emerging opportunities and challenges. We go beyond mere data presentation to offer insightful commentary on industry developments, driving forces, and potential restraints, equipping stakeholders with the knowledge needed to make informed strategic decisions.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 4.5% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 4.5%.
Key companies in the market include McKesson Corporation, Cerner Corporation, Siemens Healthcare, GE Healthcare, Allscripts, Epic System, IBM Corporation, .
The market segments include Type, Application.
The market size is estimated to be USD 112.2 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Pharmaceutical Asset Management," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Pharmaceutical Asset Management, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.