1. What is the projected Compound Annual Growth Rate (CAGR) of the Pediatric Digestive Drugs?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Pediatric Digestive Drugs
Pediatric Digestive DrugsPediatric Digestive Drugs by Type (Tablets, Pills, Oral Liquid), by Application (Hospital Pharmacies, Online Pharmacies, Retail Pharmacies), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The Pediatric Digestive Drugs market is poised for substantial growth, estimated to reach a significant market size of $9,800 million by 2025, with a projected Compound Annual Growth Rate (CAGR) of 6.5% through 2033. This expansion is fueled by a confluence of factors, including the increasing prevalence of gastrointestinal disorders in children, heightened parental awareness regarding pediatric health, and advancements in pharmaceutical research leading to more effective and targeted treatments. Rising disposable incomes in emerging economies are also contributing to better access to healthcare and specialized pediatric medications. The market is segmented into various drug types, with oral liquids expected to dominate due to ease of administration for young children, followed by tablets and pills. The application landscape is characterized by strong performance across hospital pharmacies, online pharmacies, and retail pharmacies, reflecting a diversified distribution network. Key growth drivers include the rising incidence of conditions like acid reflux, constipation, diarrhea, and inflammatory bowel diseases in pediatric populations, alongside a growing emphasis on preventative healthcare and early intervention.
The global pediatric digestive health landscape is further shaped by a dynamic set of trends and a few limiting factors. The increasing demand for novel drug formulations with improved palatability and efficacy is a significant trend, alongside the growing adoption of personalized medicine approaches tailored to individual pediatric needs. Furthermore, the focus on over-the-counter (OTC) digestive aids for minor ailments is gaining traction, expanding the accessibility of certain treatments. However, the market also faces restraints such as stringent regulatory approvals for pediatric pharmaceuticals, which can prolong development timelines and increase costs. The potential for off-label drug use and the need for robust post-market surveillance also present challenges. Despite these hurdles, the robust pipeline of innovative therapies and the unwavering commitment to improving children's digestive health by leading companies such as Pfizer, Sanofi, and AstraZeneca, are expected to sustain the market's upward trajectory. The Asia Pacific region, particularly China and India, is anticipated to emerge as a high-growth area due to its large pediatric population and expanding healthcare infrastructure.
This comprehensive report delves into the dynamic landscape of Pediatric Digestive Drugs, offering an in-depth analysis of market trends, driving forces, challenges, and future growth trajectories. Spanning a Study Period of 2019-2033, with a Base Year and Estimated Year of 2025, and a Forecast Period of 2025-2033, the report meticulously examines the Historical Period from 2019-2024. It quantifies market performance with unit sales in the millions and provides strategic insights for stakeholders navigating this vital segment of the pharmaceutical industry. The report covers a wide array of companies, including AstraZeneca, Sanofi, Aptalis, Eisai, Pfizer, Zeria (Tillotts), GlaxoSmithKline, Salix Pharmaceuticals, Perrigo, Abbott, Honz Pharmaceutical, Shandong DYNE Marine Biopharmaceutical, Sunflower Pharmaceutical, Jiangzhong Pharmaceutical, Yabao Pharmaceutical, Jumpcan Pharmaceutical, and Hansen Pharmaceutical, alongside key segments such as Tablets, Pills, Oral Liquid, and applications across Hospital Pharmacies, Online Pharmacies, and Retail Pharmacies.
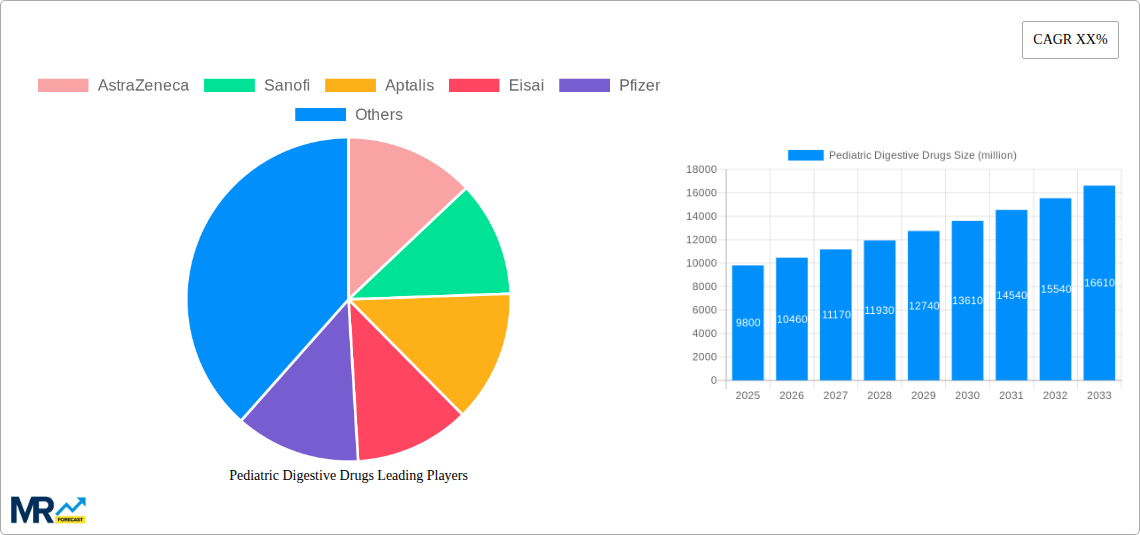
The pediatric digestive drugs market is experiencing a significant upward trajectory, driven by a confluence of factors including increasing awareness of childhood gastrointestinal disorders, advancements in diagnostic capabilities, and a growing demand for specialized formulations catering to younger populations. The market, projected to reach substantial figures in millions of units sold during the forecast period, is witnessing a shift towards more targeted and effective therapies for conditions such as acid reflux, constipation, diarrhea, and inflammatory bowel diseases in children. Insights reveal a growing preference for oral liquid formulations due to ease of administration in infants and young children, leading to a substantial unit volume in this segment. Concurrently, the increasing prevalence of lifestyle-related digestive issues, coupled with better access to healthcare services, is contributing to sustained market growth. The integration of digital platforms and e-commerce has also revolutionized the accessibility of these medications, with online pharmacies emerging as a significant distribution channel, experiencing rapid growth in unit sales. Furthermore, the rising disposable incomes in emerging economies are fueling the demand for over-the-counter (OTC) pediatric digestive remedies, further bolstering market expansion. Key trends also point towards an increasing focus on research and development for novel drug delivery systems and innovative therapeutic approaches to address the unique physiological needs of pediatric patients, aiming to improve efficacy and reduce side effects. The market is characterized by a robust pipeline of drugs targeting specific pediatric gastrointestinal conditions, promising a diversified and competitive future. The emphasis on patient convenience and improved palatability of medications is also a noteworthy trend, encouraging pharmaceutical companies to invest in developing more child-friendly drug forms. Overall, the pediatric digestive drugs market is poised for sustained and robust growth, underpinned by medical necessity, evolving consumer preferences, and ongoing innovation. The increasing incidence of pediatric obesity, often linked to digestive discomfort and metabolic imbalances, also represents a significant sub-trend contributing to the overall demand for digestive health solutions in children.
Several powerful forces are propelling the pediatric digestive drugs market forward, ensuring its continued expansion and evolution. A primary driver is the escalating incidence of gastrointestinal disorders in children worldwide. Factors such as changing dietary habits, increased consumption of processed foods, and the rise of sedentary lifestyles are contributing to a higher prevalence of conditions like acid reflux, gastritis, constipation, and Irritable Bowel Syndrome (IBS) in pediatric populations. This surge in demand for effective treatments directly fuels market growth, translating into millions of unit sales annually. Furthermore, enhanced diagnostic capabilities and a greater understanding of pediatric gastroenterology are enabling earlier and more accurate identification of digestive ailments, leading to prompt medical intervention and the prescription of appropriate medications. Pediatricians and healthcare providers are increasingly equipped to diagnose and manage a wider spectrum of digestive issues, thereby increasing the utilization of specialized pediatric digestive drugs. The growing awareness among parents regarding their children's digestive health is another significant propellant. Educated parents are more proactive in seeking medical advice and opting for treatments that can alleviate their children's discomfort and improve their overall well-being. This heightened parental concern translates into consistent demand for both prescription and over-the-counter (OTC) pediatric digestive solutions. The continuous innovation within the pharmaceutical industry, with a focus on developing safer, more effective, and child-friendly formulations, also plays a crucial role. Companies are investing heavily in research and development to create medications with improved palatability, ease of administration, and reduced side effects, making them more appealing and manageable for young patients. This commitment to innovation ensures a steady stream of new and improved therapeutic options, further stimulating market growth and contributing to millions of units sold.
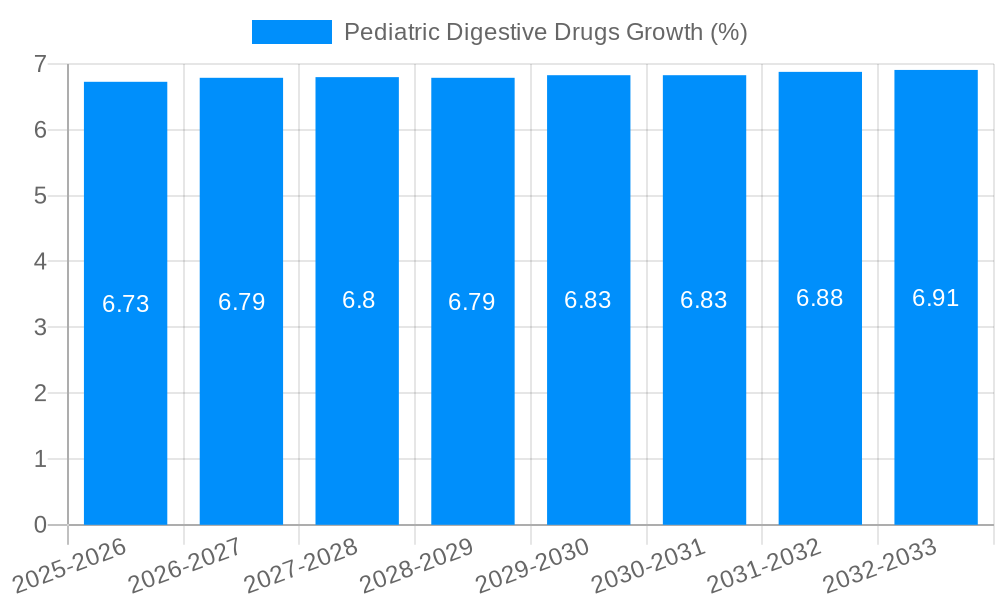
Despite the promising growth trajectory, the pediatric digestive drugs market faces several significant challenges and restraints that can impede its full potential. One of the most prominent challenges is the limited availability of drugs specifically formulated and approved for pediatric use. Historically, many drugs were developed and tested primarily on adult populations, leading to a dearth of pediatric-specific data and approved formulations. This often necessitates off-label prescribing, which carries its own set of risks and regulatory complexities. The rigorous and often lengthy process of obtaining regulatory approval for pediatric drugs presents another substantial hurdle. Clinical trials in children are complex, ethically sensitive, and require specialized methodologies, contributing to high development costs and extended timelines. This can slow down the introduction of new and innovative therapies into the market. Formulation challenges are also a significant restraint. Pediatric patients, especially infants and toddlers, have unique physiological needs and preferences. Developing palatable, easy-to-administer, and stable formulations that are acceptable to children can be a considerable undertaking. The lack of appropriate taste-masking technologies and the difficulty in ensuring consistent dosing in younger children can impact treatment adherence and overall therapeutic outcomes, thereby limiting the sales volume in millions of units for certain product types. Furthermore, the cost of pediatric-specific medications can be a restraint. The specialized nature of their development and the smaller patient populations compared to adult markets can lead to higher per-unit costs, potentially limiting accessibility for some families, especially in regions with limited healthcare infrastructure or lower disposable incomes. Concerns regarding the long-term safety and efficacy of certain digestive drugs in developing children also warrant careful consideration and can lead to cautious prescribing practices. The potential for adverse effects on growth and development requires ongoing monitoring and robust post-market surveillance, adding another layer of complexity to market dynamics. Finally, evolving regulatory landscapes and stringent quality control requirements for pharmaceuticals, particularly those intended for children, necessitate continuous investment in compliance and quality assurance, which can add to operational costs and impact market entry for smaller players.
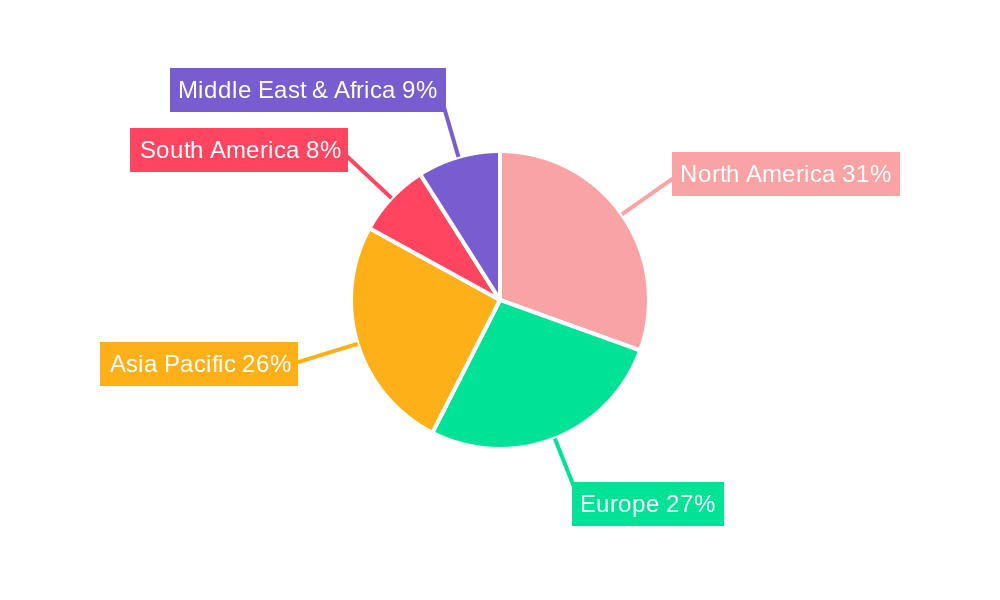
The pediatric digestive drugs market exhibits distinct regional dominance and segment popularity, driven by a complex interplay of healthcare infrastructure, economic factors, regulatory environments, and disease prevalence.
Key Regions/Countries Dominating the Market:
North America (United States and Canada): This region consistently leads the market due to several compelling factors. The high prevalence of gastrointestinal disorders in children, coupled with a well-established healthcare system and significant disposable income, drives substantial demand for pediatric digestive drugs. The presence of major pharmaceutical companies like Pfizer and Abbott, with robust research and development capabilities focused on pediatric pharmaceuticals, further solidifies North America's leading position. Advanced diagnostic technologies and a high rate of physician adoption of new treatments contribute to the significant unit sales in millions across various drug categories. Moreover, the strong emphasis on parental health education and proactive healthcare seeking behavior among the population ensures sustained market penetration. The robust reimbursement policies and insurance coverage for a wide range of prescription medications also facilitate higher sales volumes.
Europe (Germany, United Kingdom, France, Italy, Spain): The European market represents another significant powerhouse, characterized by a strong healthcare infrastructure, increasing awareness of pediatric gastrointestinal health, and a growing preference for evidence-based medicine. Countries like Germany and the UK, with their advanced healthcare systems and proactive regulatory bodies, witness substantial demand for a broad spectrum of pediatric digestive drugs. The presence of pharmaceutical giants such as AstraZeneca and Sanofi, with dedicated pediatric divisions, contributes to market dynamism. The rising incidence of lifestyle-related digestive issues in children across Europe, mirroring trends in North America, further fuels market growth. The availability of both prescription and over-the-counter (OTC) options, coupled with increasing accessibility through hospital pharmacies and a growing online pharmacy presence, ensures consistent unit sales in millions.
Asia-Pacific (China, Japan, India, South Korea): This region is emerging as a high-growth market, driven by a rapidly expanding population, increasing healthcare expenditure, and a growing awareness of childhood health issues. China, in particular, is a significant contributor due to its vast pediatric population and the increasing adoption of advanced medical treatments. Companies like Jiangzhong Pharmaceutical and Yabao Pharmaceutical are key players in this region, catering to the immense demand. The rising disposable incomes and the government's focus on improving healthcare access are further bolstering the pediatric digestive drugs market. While traditionally reliant on generics, there is a growing demand for branded and specialized pediatric formulations, leading to an increase in unit sales in millions. Japan and South Korea also contribute significantly with their advanced pharmaceutical sectors and high standards of healthcare.
Key Segment Dominating the Market:
Several key factors are acting as significant growth catalysts for the pediatric digestive drugs industry. The rising global incidence of gastrointestinal disorders in children, fueled by changing lifestyles and dietary habits, is a primary driver. Increased parental awareness regarding the importance of children's digestive health and a proactive approach to seeking medical solutions further bolsters demand. Moreover, advancements in pharmaceutical research and development are leading to the creation of more effective, safer, and child-friendly formulations, including improved taste-masking technologies and easier administration methods. The expansion of healthcare infrastructure, particularly in emerging economies, and greater accessibility to pediatric specialists are also crucial catalysts.
This report offers a panoramic view of the pediatric digestive drugs market, providing stakeholders with invaluable insights for strategic decision-making. It meticulously dissects market trends, identifying key growth areas and potential challenges. The analysis extends to the underlying drivers of market expansion, such as increasing disease prevalence and heightened parental awareness, alongside significant restraints like regulatory hurdles and formulation complexities. Detailed segmentation by drug type and application channels, including hospital, online, and retail pharmacies, highlights areas of opportunity and evolving consumer preferences. Furthermore, the report profiles leading industry players and chronicles significant developmental milestones. By combining quantitative data on unit sales in millions with qualitative market intelligence, this report empowers businesses to navigate the dynamic pediatric digestive drugs landscape, optimize their strategies, and capitalize on future growth prospects.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include AstraZeneca, Sanofi, Aptalis, Eisai, Pfizer, Zeria (Tillotts), GlaxoSmithKline, Salix Pharmaceuticals, Perrigo, Abbott, Honz Pharmaceutical, Shandong DYNE Marine Biopharmaceutical, Sunflower Pharmaceutical, Jiangzhong Pharmaceutical, Yabao Pharmaceutical, Jumpcan Pharmaceutical, Hansen Pharmaceutical.
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Pediatric Digestive Drugs," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Pediatric Digestive Drugs, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.