1. What is the projected Compound Annual Growth Rate (CAGR) of the Non-Invasive Vagus Nerve Stimulation Device?
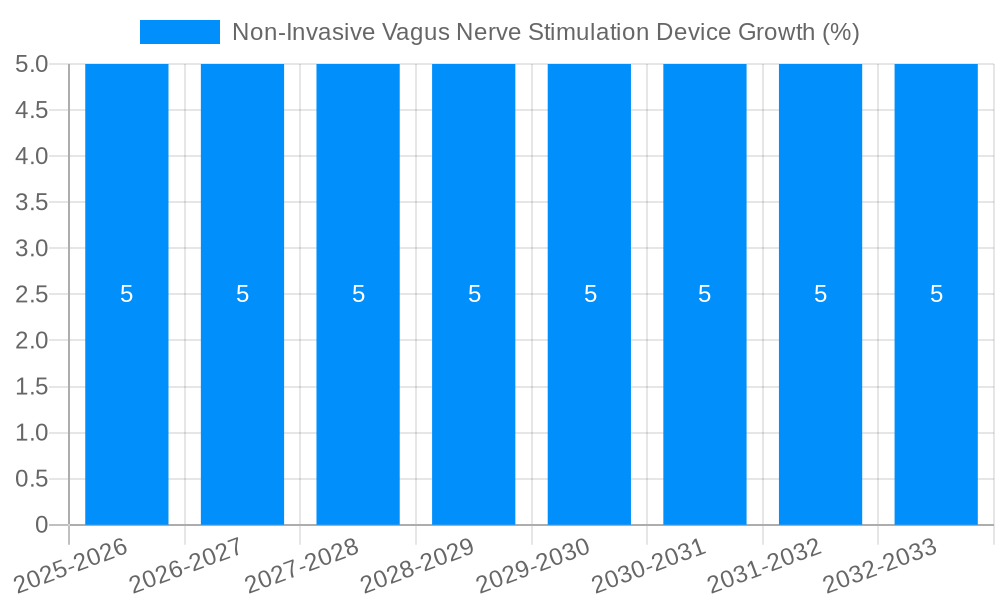
The projected CAGR is approximately 5%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Non-Invasive Vagus Nerve Stimulation Device
Non-Invasive Vagus Nerve Stimulation DeviceNon-Invasive Vagus Nerve Stimulation Device by Type (Depression, Epilepsy, Others, World Non-Invasive Vagus Nerve Stimulation Device Production ), by Application (Hospitals, Ambulatory Surgical Centers, Others, World Non-Invasive Vagus Nerve Stimulation Device Production ), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The global Non-Invasive Vagus Nerve Stimulation Device market is poised for significant expansion, projected to reach a valuation of $1211.9 million by 2025 and continuing its growth trajectory with a compound annual growth rate (CAGR) of 5% through 2033. This robust growth is primarily fueled by an increasing prevalence of neurological and psychiatric disorders, such as depression and epilepsy, which are becoming more widely diagnosed and demanding advanced treatment options. The inherent advantages of non-invasive neuromodulation techniques, including minimal side effects, improved patient compliance, and a less burdensome treatment protocol compared to surgical interventions, are driving adoption. Furthermore, advancements in technology, leading to more sophisticated and user-friendly devices, coupled with growing healthcare expenditure and an aging global population susceptible to these conditions, are strong market enablers.
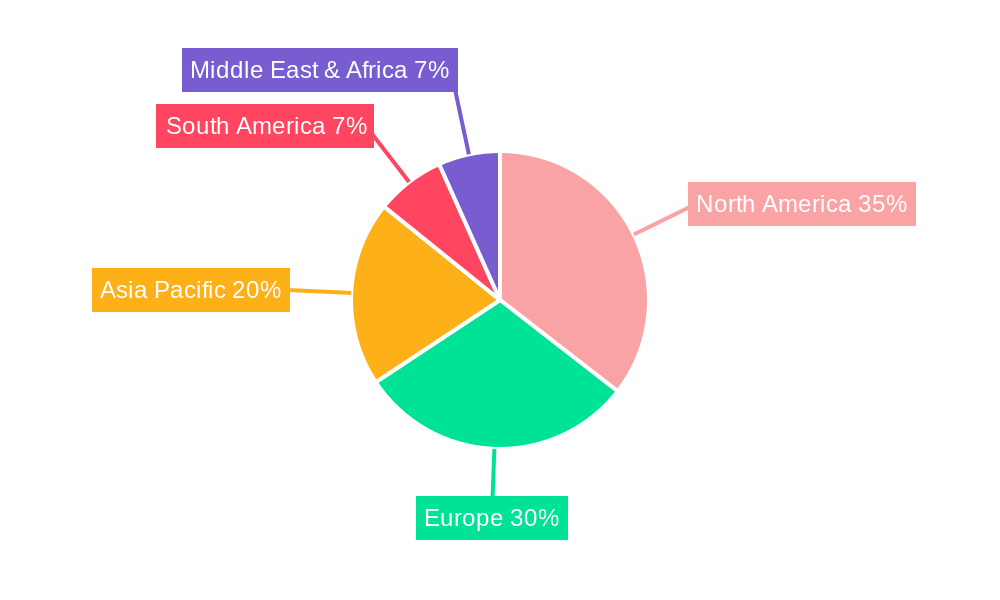
The market segmentation reveals a diverse landscape, with "Depression" and "Epilepsy" emerging as key application areas, indicating a concentrated demand for devices addressing these prevalent disorders. While hospitals are expected to remain primary adoption centers due to established infrastructure for advanced medical devices, ambulatory surgical centers are likely to witness increasing utilization as treatment protocols become more streamlined and accessible. The competitive landscape is characterized by the presence of established players like Cyberonics, Spark Biomedical, and Nevro Corporation, alongside emerging innovators such as Galvani Bioelectronics. These companies are actively investing in research and development to enhance device efficacy, explore new therapeutic applications, and expand their geographical reach, particularly in North America and Europe, which are anticipated to dominate market share due to strong healthcare systems and higher disposable incomes.
This comprehensive report delves into the intricate dynamics of the global Non-Invasive Vagus Nerve Stimulation (NIVNS) device market, offering an in-depth analysis spanning the historical period of 2019-2024, the estimated base year of 2025, and projecting significant growth through the forecast period of 2025-2033. With a keen focus on key market insights, driving forces, challenges, regional dominance, growth catalysts, leading players, and significant industry developments, this report provides a critical understanding of this burgeoning therapeutic modality. The analysis incorporates production and application data in the millions of units, offering a quantitative perspective on market evolution.
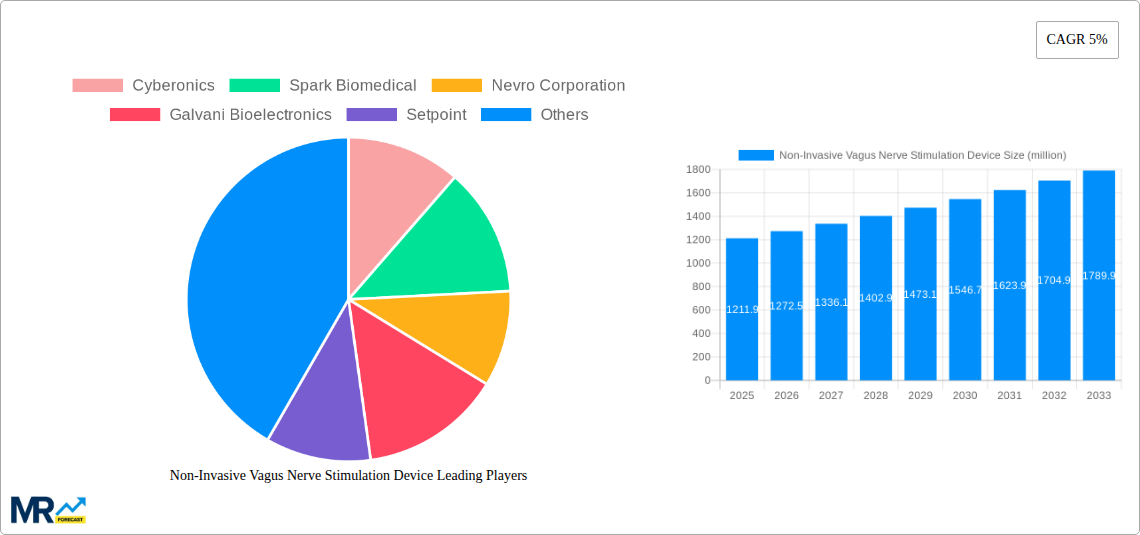
The Non-Invasive Vagus Nerve Stimulation (NIVNS) device market is experiencing a transformative surge, driven by increasing patient demand for less invasive treatment options and a growing body of scientific evidence supporting its efficacy across a spectrum of neurological and psychiatric conditions. During the historical period from 2019 to 2024, the market witnessed steady growth, fueled by initial regulatory approvals and early adoption by healthcare providers. The estimated market in the base year of 2025 is projected to be a significant contributor to the overall healthcare expenditure, with production volumes reaching into the millions of units. The forecast period, from 2025 to 2033, anticipates an accelerated growth trajectory, propelled by advancements in device technology, expansion into new therapeutic areas, and a broader acceptance by the medical community. Key trends include the increasing miniaturization of devices, enhanced customization of stimulation parameters to optimize patient outcomes, and the integration of artificial intelligence for personalized treatment algorithms. Furthermore, the market is observing a growing trend towards home-use NIVNS devices, empowering patients with greater autonomy and reducing the burden on clinical settings. The expansion of reimbursement policies and increased insurance coverage for NIVNS therapies are also critical trends shaping market accessibility and adoption. The growing awareness campaigns highlighting the benefits of vagus nerve stimulation, particularly for conditions that are often refractory to conventional treatments, are further contributing to its rising prominence. The research landscape is also a key indicator of future trends, with ongoing studies exploring NIVNS for conditions such as chronic pain, inflammatory disorders, and even cognitive enhancement, suggesting a vast untapped potential for future market expansion. The production of NIVNS devices, measured in millions of units, is expected to see a substantial uptick as demand scales and manufacturing processes become more efficient. The application of these devices is also diversifying, moving beyond established uses in epilepsy and depression towards broader neurological and psychiatric indications.
The Non-Invasive Vagus Nerve Stimulation (NIVNS) device market's robust growth is underpinned by a confluence of powerful driving forces. Foremost among these is the escalating global prevalence of neurological and psychiatric disorders, including depression, epilepsy, and anxiety, which are creating an unmet need for effective and accessible treatment modalities. NIVNS offers a compelling alternative to more invasive surgical interventions, significantly reducing patient risk and recovery time. This inherent safety profile, coupled with a growing body of clinical evidence demonstrating the efficacy of NIVNS in managing symptoms of these debilitating conditions, is a primary driver for its adoption. The continuous technological advancements in NIVNS devices, leading to improved precision, user-friendliness, and affordability, are also critical in expanding market reach. Furthermore, increasing patient awareness and a growing preference for non-pharmacological or adjunctive therapies are contributing to higher demand. The supportive regulatory landscape in many regions, facilitating faster approval pathways for innovative NIVNS technologies, is another significant propellant. Investments in research and development by key market players, aimed at exploring new applications and refining existing technologies, are consistently fueling innovation and market expansion. The economic burden associated with chronic neurological and psychiatric conditions also encourages the exploration of cost-effective treatment solutions like NIVNS, which can potentially reduce long-term healthcare costs associated with hospitalizations and managing complex symptoms. The shift towards personalized medicine, where treatments are tailored to individual patient needs, is also a significant driver, as NIVNS allows for customizable stimulation parameters.
Despite its promising trajectory, the Non-Invasive Vagus Nerve Stimulation (NIVNS) device market faces several significant challenges and restraints that could temper its growth. A primary hurdle is the perceived cost of NIVNS devices and the associated treatment protocols. While generally less expensive than surgical alternatives, the initial investment and ongoing therapy costs can still be prohibitive for a substantial portion of the patient population, particularly in regions with limited healthcare coverage. The lack of widespread reimbursement policies from insurance providers in certain geographies remains a critical barrier to market penetration, limiting patient access and physician adoption. Educating both healthcare professionals and the general public about the benefits, mechanisms, and proper usage of NIVNS devices is an ongoing challenge. Misconceptions and a lack of familiarity with this relatively newer therapeutic modality can lead to hesitation and slower uptake. Furthermore, the efficacy of NIVNS can vary significantly among individuals, and identifying optimal stimulation parameters for each patient requires expertise and iterative adjustments, which can be resource-intensive. The current regulatory landscape, while generally supportive, can still present complexities and lengthy approval processes for novel NIVNS technologies and expanded indications, thereby delaying market entry. Long-term efficacy data and large-scale, multi-center clinical trials are still being gathered for some applications, which can create a perception of uncertainty for some clinicians and payers. The competitive landscape, with the presence of both established neuromodulation companies and emerging innovators, can lead to pricing pressures and challenges in differentiating product offerings. Finally, the need for patient adherence and commitment to daily or regular stimulation sessions can also be a restraining factor for individuals who find consistent usage challenging.
The global Non-Invasive Vagus Nerve Stimulation (NIVNS) device market is poised for substantial growth, with certain regions and application segments expected to lead this expansion. Among the segments, Depression is projected to be a dominant force in the market. The staggering global burden of depressive disorders, coupled with the increasing recognition of NIVNS as a viable treatment option for medication-resistant depression, positions this segment for significant market share. The increasing research and clinical trials focusing on NIVNS for various forms of depression, from major depressive disorder to treatment-resistant depression, are continuously expanding the therapeutic landscape. Patient demand for non-pharmacological and adjunctive treatments for depression, particularly those with fewer side effects than traditional antidepressants, is a key driver. The United States is anticipated to emerge as a dominant region in the NIVNS market. Several factors contribute to this regional leadership:
In terms of Application, Hospitals are expected to remain a primary segment for NIVNS device deployment, given their established infrastructure for patient care and the need for specialized medical supervision during initial treatment phases. However, the Others application segment, encompassing specialized neurological clinics, outpatient treatment centers, and potentially home-use scenarios, is projected for significant growth. This is driven by the increasing trend towards decentralizing healthcare delivery and empowering patients with self-management tools. The production of NIVNS devices, measured in millions of units, will be concentrated in regions with robust manufacturing capabilities and a strong presence of key players.
Several key growth catalysts are expected to propel the Non-Invasive Vagus Nerve Stimulation (NIVNS) device industry forward. The most significant catalyst is the expanding clinical evidence base, demonstrating the efficacy of NIVNS across an ever-wider range of indications, particularly in treating refractory depression and epilepsy. This growing body of scientific validation is crucial in building clinician confidence and encouraging broader adoption. Furthermore, advancements in device miniaturization and user-friendliness are making NIVNS more accessible and appealing to patients, fostering greater adherence and facilitating its use in diverse settings, including home-based therapies. The increasing prevalence of target neurological and psychiatric conditions globally ensures a continuously expanding patient population seeking novel treatment solutions. Finally, the supportive regulatory environments and the pursuit of favorable reimbursement policies by key market players are vital in reducing cost barriers and enhancing market penetration.
This report offers unparalleled comprehensive coverage of the Non-Invasive Vagus Nerve Stimulation (NIVNS) device market. It meticulously analyzes market dynamics from the historical period of 2019-2024, establishes a critical base year estimate for 2025, and provides detailed projections for the forecast period of 2025-2033. The report dissects the market by production volume in millions of units and categorizes applications across Hospitals, Ambulatory Surgical Centers, and Others. It further segments the market by therapeutic type, including Depression, Epilepsy, and Others. Leading players such as Cyberonics, Spark Biomedical, Nevro Corporation, Galvani Bioelectronics, Setpoint, Electrocore, Livanova, MicroTransponder, CVRx, and Beijing PINS Medical are thoroughly profiled. Significant industry developments, including key technological advancements and regulatory milestones, are tracked and analyzed. This comprehensive approach ensures stakeholders gain a complete understanding of the market's present state, future potential, and the intricate factors shaping its evolution.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 5%.
Key companies in the market include Cyberonics, Spark Biomedical, Nevro Corporation, Galvani Bioelectronics, Setpoint, Electrocore, Livanova, MicroTransponder, CVRx, Beijing PINS Medical, .
The market segments include Type, Application.
The market size is estimated to be USD 1211.9 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Non-Invasive Vagus Nerve Stimulation Device," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Non-Invasive Vagus Nerve Stimulation Device, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.