1. What is the projected Compound Annual Growth Rate (CAGR) of the Needle-Free Devices?
The projected CAGR is approximately 5.1%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Needle-Free Devices
Needle-Free DevicesNeedle-Free Devices by Type (Spring-Loaded Jet Injector, Battery-Powered Jet Injector, Gas-Powered Jet Injector), by Application (Hospitals, Clinics, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
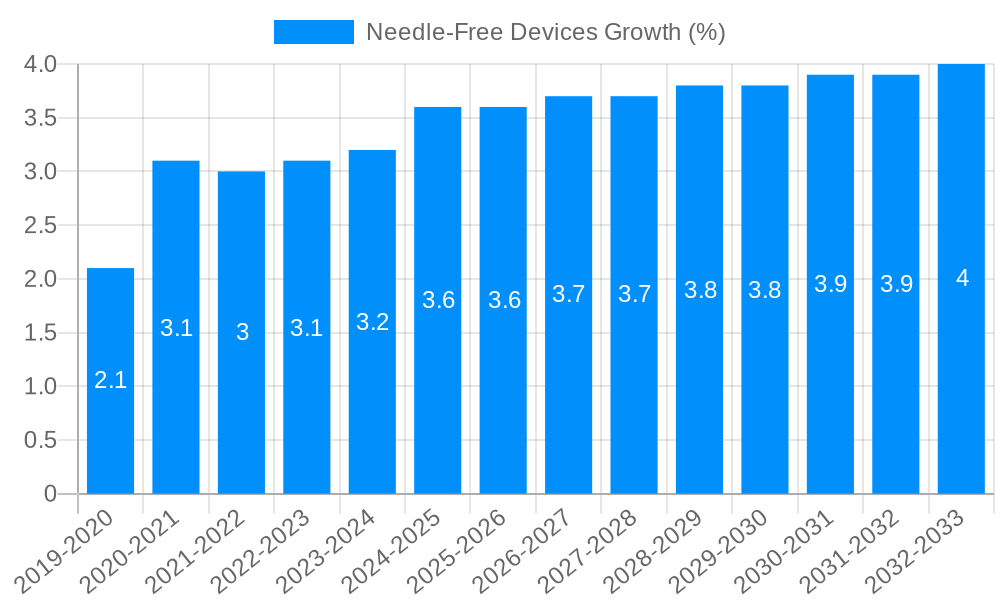
The global Needle-Free Devices market is poised for significant expansion, projected to reach approximately $2813.1 million by 2025, with a robust Compound Annual Growth Rate (CAGR) of 5.1% anticipated to persist through 2033. This growth is primarily fueled by the increasing adoption of advanced drug delivery systems, driven by a growing demand for less invasive and more patient-friendly administration methods, especially for chronic conditions and vaccinations. The inherent advantages of needle-free technology, such as reduced pain, fear of needles, and the elimination of needlestick injuries, are key drivers accelerating market penetration across healthcare settings. Technological advancements in miniaturization, improved efficacy, and enhanced user experience are continually pushing the boundaries of what these devices can achieve, further bolstering market demand.
The market is segmented by type into Spring-Loaded Jet Injectors, Battery-Powered Jet Injectors, and Gas-Powered Jet Injectors, with each segment catering to specific application needs and patient demographics. Hospitals and clinics represent the dominant application segments, owing to their extensive use in routine patient care, vaccination drives, and the administration of specialized medications. However, the "Others" segment, encompassing home healthcare and specialized medical facilities, is also expected to witness notable growth as awareness and accessibility of needle-free solutions increase. Key players such as Pfizer, Antares Pharma, Vygon (UK) Ltd, Romsons, and BD are actively investing in research and development, strategic collaborations, and market expansion initiatives to capitalize on these burgeoning opportunities, further solidifying the market's upward trajectory and contributing to its dynamic evolution.
This report delves into the dynamic and rapidly evolving needle-free devices market, providing a comprehensive analysis of trends, drivers, challenges, and future growth prospects. Spanning a study period from 2019 to 2033, with a base year of 2025, the report meticulously examines the historical trajectory, current market landscape, and projected advancements in this crucial healthcare sector. Through in-depth research and robust data, the report offers invaluable insights for stakeholders seeking to understand the market's nuances and identify strategic opportunities. It will shed light on the innovative technologies that are redefining drug delivery, enhancing patient comfort, and improving therapeutic outcomes.
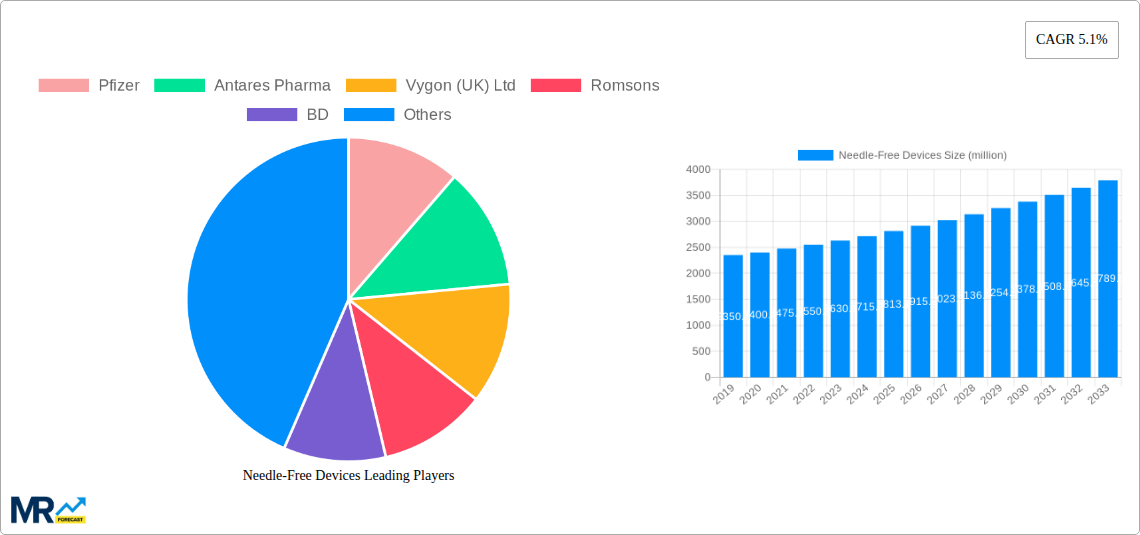
The needle-free devices market is experiencing a significant upward trajectory, driven by an escalating demand for minimally invasive drug delivery systems and a growing awareness of the limitations and anxieties associated with traditional needle injections. During the historical period of 2019-2024, the market witnessed steady growth, laying the foundation for more substantial expansion. The estimated year of 2025 marks a pivotal point, with the market projected to reach over 800 million units in global sales. This growth is intrinsically linked to the increasing prevalence of chronic diseases requiring regular medication, such as diabetes, autoimmune disorders, and allergies, where repeated injections are often a necessity. Furthermore, the pediatric population, often expressing considerable fear of needles, is a key demographic that benefits immensely from these advanced delivery mechanisms, leading to higher adoption rates in settings catering to children.
The forecast period of 2025-2033 is anticipated to witness an accelerated growth curve. This surge will be fueled by continuous technological innovation, leading to more sophisticated and user-friendly needle-free devices. The development of smart devices that can track dosage, adherence, and even provide real-time feedback to patients and healthcare providers will become increasingly common. The integration of artificial intelligence and personalized medicine approaches will further enhance the efficacy and patient experience. Moreover, the expanding indications for needle-free delivery, moving beyond vaccines and insulin to include biologics and complex drug formulations, will broaden the market's scope. The sheer volume of therapeutic interventions that can be made more palatable and accessible through needle-free technologies underscores its immense potential. The market's projected expansion to well over 1.5 billion units by 2033 signifies a profound shift in drug delivery paradigms.
The needle-free devices market is being propelled by a confluence of factors that underscore the increasing preference for safer, more comfortable, and more effective drug administration methods. A primary driver is the growing global burden of chronic diseases, necessitating regular and often lifelong medication. Conditions such as diabetes, rheumatoid arthritis, and multiple sclerosis require frequent subcutaneous or intramuscular injections, and needle-free devices offer a compelling alternative to traditional syringes, significantly enhancing patient compliance and quality of life. The inherent fear and pain associated with needle injections, particularly among children and individuals with needle phobia, are substantial deterrents to consistent medication adherence. Needle-free technologies effectively circumvent this issue, promoting a more positive patient experience and leading to improved health outcomes. Furthermore, the increasing focus on infection control in healthcare settings, with a particular emphasis on preventing needle-stick injuries among healthcare professionals, further bolsters the demand for needle-free solutions. The inherent safety profile of these devices in this regard is a significant advantage, reducing the risk of accidental punctures and subsequent transmission of blood-borne pathogens.
Despite the promising growth trajectory, the needle-free devices market is not without its challenges and restraints. A significant hurdle is the higher initial cost associated with many needle-free devices compared to conventional syringes and needles. This price differential can be a barrier to widespread adoption, especially in resource-limited healthcare settings and for self-pay patients. While the long-term benefits of improved compliance and reduced complications can outweigh the initial cost, the upfront investment remains a point of concern. Another challenge lies in the varying efficacy and suitability of different needle-free technologies for diverse drug formulations and viscosities. Not all needle-free devices are universally compatible with every type of medication, requiring specific device-drug pairings. This necessitates ongoing research and development to broaden the applicability of these devices. Furthermore, regulatory hurdles and the need for extensive clinical trials to demonstrate safety and efficacy for new needle-free delivery systems can prolong market entry timelines and increase development costs. Patient and healthcare provider education also plays a crucial role; a lack of familiarity or understanding of how to use these devices correctly can lead to suboptimal outcomes and hinder widespread acceptance.
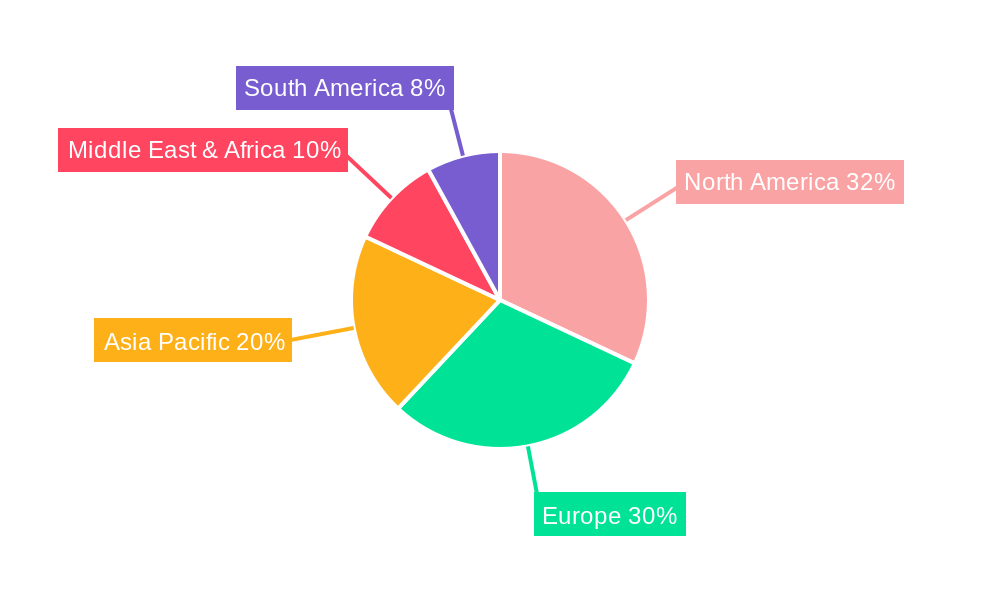
The needle-free devices market is poised for significant dominance by specific regions and segments, driven by a complex interplay of healthcare infrastructure, patient demographics, and technological adoption. North America is anticipated to be a leading region, primarily due to its advanced healthcare system, high disposable incomes, and a strong emphasis on patient comfort and technological innovation. The substantial prevalence of chronic diseases like diabetes and autoimmune disorders, coupled with a proactive approach to adopting novel medical technologies, positions North America at the forefront of market expansion. The region's well-established pharmaceutical and medical device industries, with key players like Pfizer and Antares Pharma, further bolster this dominance by investing heavily in research and development of needle-free solutions.
Within the diverse segments of the needle-free devices market, the Hospitals application segment is projected to hold a substantial share. This is attributed to the high volume of drug administration in hospital settings, including vaccinations, chronic disease management, and emergency treatments. The increasing focus on infection control and the desire to minimize needlestick injuries among healthcare professionals further drive the adoption of needle-free devices in hospitals. Moreover, hospitals are often early adopters of advanced medical technologies due to their access to funding and their commitment to providing the highest standard of patient care.
Among the different types of needle-free devices, Battery-Powered Jet Injectors are expected to witness the most significant growth and potentially dominate the market in the coming years. This segment's ascendancy is driven by their versatility, precision, and user-friendliness. Battery-powered devices offer greater control over the injection process, allowing for adjustable dosage and injection depth, which is crucial for a wide range of medications and patient types. Their rechargeable nature also makes them more cost-effective and environmentally friendly in the long run compared to disposable cartridges or single-use spring mechanisms. The technological advancements in battery life, miniaturization, and smart functionalities, such as data logging and connectivity, are further enhancing the appeal of battery-powered jet injectors. Companies like Vygon (UK) Ltd are actively contributing to this segment's growth with their innovative product offerings.
The Clinics segment, encompassing various outpatient facilities, is also expected to demonstrate robust growth. As healthcare systems increasingly shift towards outpatient care and remote patient monitoring, clinics will become central hubs for medication delivery. Needle-free devices are ideal for clinic settings due to their ease of use, reduced training requirements for staff, and improved patient experience during routine treatments. The growing trend of home healthcare and self-administration of medications will also indirectly boost the clinic segment, as patients might be trained on using needle-free devices in clinics before transitioning to home use.
The "Others" segment, which could include specialized settings like pharmacies offering vaccination services, corporate wellness programs, and even direct-to-consumer channels for certain self-administered medications, will also contribute to market diversification. As awareness and acceptance of needle-free technologies increase, their application will expand beyond traditional healthcare confines.
Geographically, while North America is expected to lead, the Asia Pacific region is poised for rapid growth. Factors contributing to this include the increasing prevalence of chronic diseases, a burgeoning middle class with greater purchasing power, and government initiatives to improve healthcare access and quality. Countries like China and India, with their vast populations, represent significant untapped potential for needle-free device adoption.
The needle-free devices industry is propelled by several key growth catalysts. The escalating global demand for minimally invasive drug delivery solutions, driven by patient preference for comfort and reduced pain, is a primary catalyst. The rising incidence of chronic diseases necessitating frequent injections, such as diabetes and autoimmune conditions, further fuels adoption. Technological advancements, leading to more sophisticated, user-friendly, and versatile devices, also play a crucial role. Furthermore, increasing awareness campaigns and educational initiatives by healthcare providers and manufacturers are enhancing patient and clinician acceptance, paving the way for broader market penetration.
This comprehensive report provides an exhaustive analysis of the needle-free devices market, encompassing market size, growth trends, competitive landscape, and future projections. It meticulously details market segmentation by type, application, and region, offering deep insights into the dynamics of each category. The report highlights key industry developments, technological innovations, and the strategic initiatives of leading market players. With an estimated market value poised to exceed over 800 million units in 2025 and projected to reach well over 1.5 billion units by 2033, this report serves as an indispensable resource for manufacturers, investors, healthcare providers, and policymakers seeking to navigate and capitalize on the burgeoning opportunities within the needle-free devices sector.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5.1% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 5.1%.
Key companies in the market include Pfizer, Antares Pharma, Vygon (UK) Ltd, Romsons, BD, .
The market segments include Type, Application.
The market size is estimated to be USD 2813.1 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Needle-Free Devices," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Needle-Free Devices, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.