1. What is the projected Compound Annual Growth Rate (CAGR) of the MicroRNA Detection Kit?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 MicroRNA Detection Kit
MicroRNA Detection KitMicroRNA Detection Kit by Type (PCR Detection Kit, RT-PCR Detection Kit, World MicroRNA Detection Kit Production ), by Application (Hospital, Clinic, Others, World MicroRNA Detection Kit Production ), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
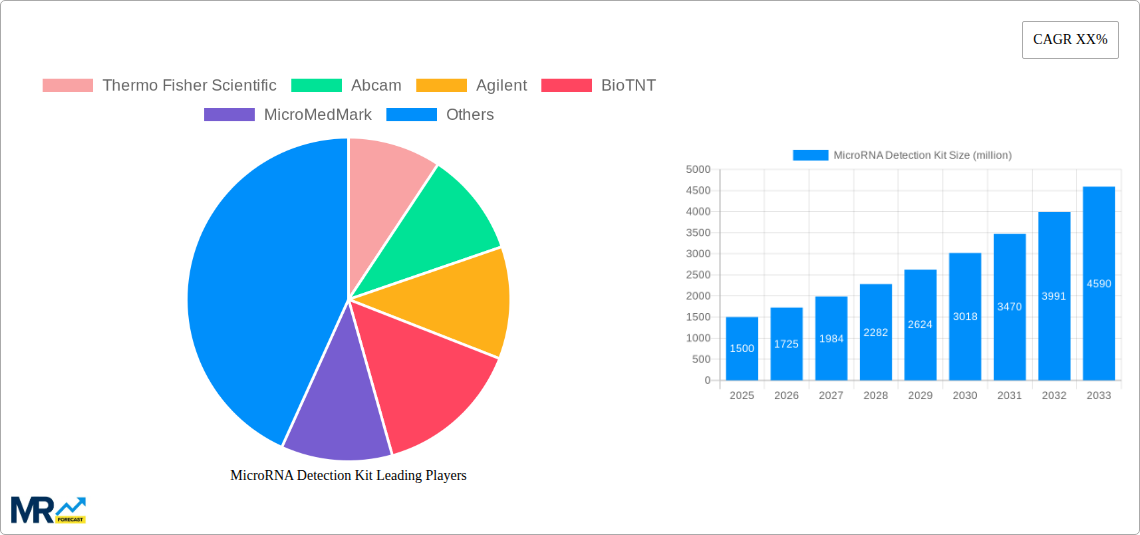
The global MicroRNA Detection Kit market is poised for significant expansion, projected to reach an estimated value of $1,500 million by 2025, with a robust Compound Annual Growth Rate (CAGR) of approximately 15% anticipated over the forecast period from 2025 to 2033. This remarkable growth is fueled by several key drivers, including the increasing prevalence of chronic diseases and infectious diseases, which necessitate more accurate and rapid diagnostic tools. The rising investment in research and development by leading biotechnology firms, coupled with advancements in molecular diagnostic technologies like Polymerase Chain Reaction (PCR) and Real-Time PCR (RT-PCR), are further propelling market adoption. The expanding applications of microRNA detection in personalized medicine, oncology, and cardiovascular disease research are also contributing to a surge in demand. Furthermore, the growing awareness among healthcare professionals and patients about the potential of microRNAs as biomarkers for early disease detection and prognosis is a critical factor driving market penetration.
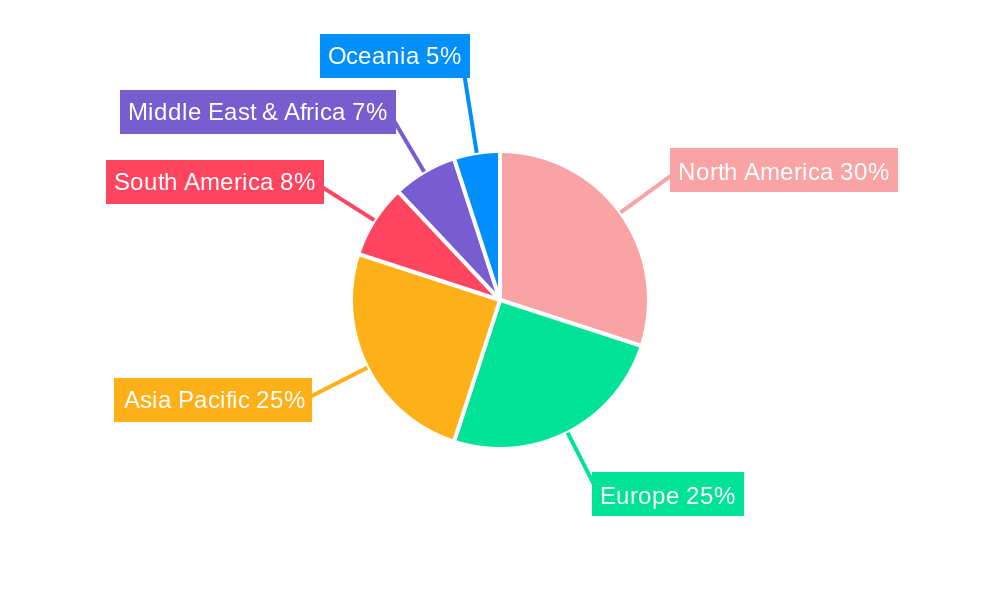
The market landscape is characterized by a diverse range of players, including giants like Thermo Fisher Scientific and Abcam, alongside specialized companies such as BioTNT and MiRXES, all contributing to the innovation and accessibility of microRNA detection solutions. The market is segmented by type, with PCR Detection Kits and RT-PCR Detection Kits dominating the current offerings, while the emerging World MicroRNA Detection Kit Production segment holds substantial future potential. Applications span across hospitals and clinics, with a growing adoption in research laboratories and diagnostic centers. Geographically, the Asia Pacific region, particularly China and India, is emerging as a high-growth area due to increasing healthcare expenditure and a burgeoning research infrastructure. North America and Europe continue to be significant markets, driven by well-established healthcare systems and a strong emphasis on advanced diagnostics. The market, however, faces some restraints, including the high cost of advanced detection technologies and the need for standardized protocols to ensure reproducibility and comparability of results across different platforms.
The global microRNA detection kit market is poised for substantial expansion, driven by an increasing understanding of microRNAs’ multifaceted roles in disease pathogenesis and their potential as diagnostic and prognostic biomarkers. During the Study Period (2019-2033), this market is anticipated to witness robust compound annual growth rates, with the Base Year (2025) serving as a critical benchmark for future projections. The Forecast Period (2025-2033) is expected to be a period of dynamic innovation and market penetration, building upon the foundational advancements observed in the Historical Period (2019-2024). By Estimated Year (2025), the market is projected to reach a valuation in the hundreds of millions of dollars, with significant revenue streams generated from both established and emerging players. The growing emphasis on personalized medicine and the demand for early disease detection tools are major contributors to this upward trajectory. Furthermore, advancements in molecular biology techniques, particularly in the realm of amplification and detection technologies, are continuously improving the sensitivity, specificity, and speed of microRNA detection, thereby fueling market adoption across various healthcare settings. The integration of microRNA analysis into routine clinical diagnostics for conditions such as various cancers, cardiovascular diseases, and neurological disorders is gradually becoming a reality. This trend is supported by ongoing research demonstrating the diagnostic power of specific microRNA signatures. The increasing availability of sophisticated and user-friendly detection kits is lowering the barrier to entry for laboratories, further stimulating market growth. Moreover, the burgeoning field of liquid biopsy, which utilizes circulating microRNAs for non-invasive disease detection, represents a significant growth avenue for the microRNA detection kit market. The development of highly sensitive and multiplexed detection platforms capable of identifying a broad spectrum of microRNAs from limited biological samples is a key area of focus for manufacturers. The market is also influenced by strategic collaborations between diagnostic companies, research institutions, and reagent providers to accelerate the translation of microRNA research into clinical applications. The expanding research landscape, particularly in oncology, where microRNAs are implicated in tumor initiation, progression, and metastasis, continues to drive the demand for reliable detection tools. As regulatory frameworks adapt to accommodate novel diagnostic technologies, the pathway for microRNA detection kits to gain widespread clinical acceptance is becoming clearer.
The microRNA detection kit market is experiencing a surge in demand propelled by several compelling factors. Foremost among these is the escalating prevalence of chronic diseases, including various cancers, cardiovascular ailments, and neurodegenerative disorders. MicroRNAs have emerged as pivotal regulators in these disease pathways, making them attractive targets for diagnostic and prognostic applications. The increasing recognition of microRNAs as early biomarkers for disease onset and progression is a significant driver, enabling earlier intervention and potentially improving patient outcomes. This is further amplified by the growing trend towards personalized medicine, where microRNA profiling can help tailor treatment strategies to individual patient needs and predict therapeutic responses. The advancement of molecular diagnostic technologies, such as quantitative polymerase chain reaction (qPCR) and next-generation sequencing (NGS), has provided more sensitive, specific, and high-throughput methods for microRNA detection, thereby increasing the utility and accessibility of these kits. Furthermore, the development of microRNA detection kits specifically designed for liquid biopsies, which allow for non-invasive sample collection (e.g., from blood or urine), is revolutionizing disease monitoring and early detection. This approach circumvents the need for invasive tissue biopsies, making diagnostics more patient-friendly and cost-effective. The robust investment in life sciences research and development, particularly in identifying novel microRNA biomarkers for a wide range of diseases, continues to expand the potential applications and thus the market for these detection kits.
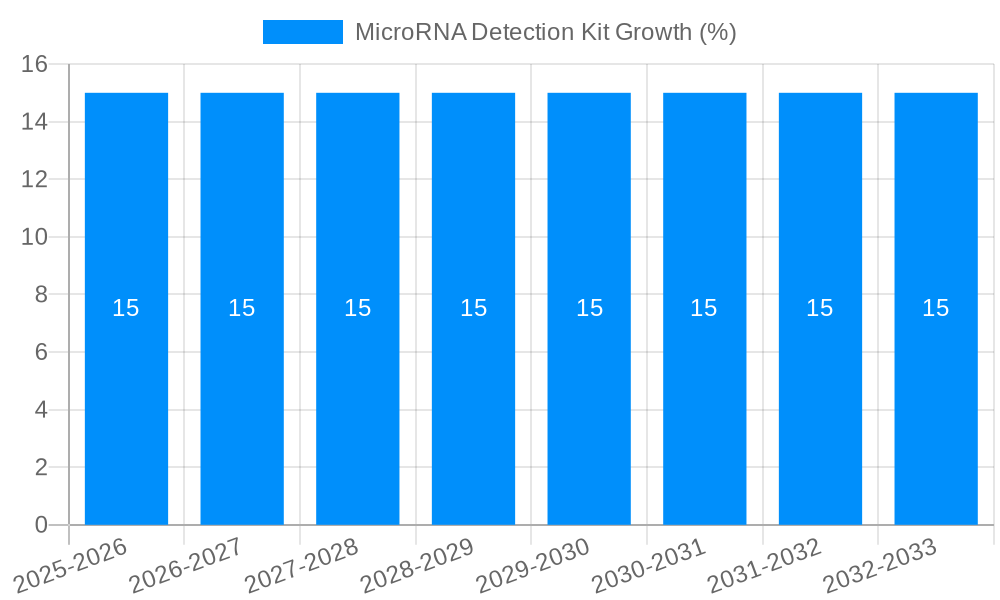
Despite the promising growth trajectory, the microRNA detection kit market faces several significant challenges and restraints that can impede its full potential. A primary hurdle is the inherent complexity and heterogeneity of microRNA biology. Understanding the precise role and accurate quantification of thousands of microRNAs within a given biological context remains a complex task. This complexity can lead to difficulties in identifying truly reliable and universally applicable microRNA biomarkers. Standardization of detection methods and data analysis protocols across different laboratories and platforms is another critical issue. Variations in sample preparation, RNA extraction efficiency, and assay methodologies can lead to inconsistent and non-reproducible results, hindering the clinical validation and adoption of microRNA detection kits. The cost of advanced detection technologies and reagents, while decreasing, can still be a barrier for widespread implementation, particularly in resource-limited settings. Furthermore, the regulatory landscape for novel diagnostic tools like microRNA detection kits can be lengthy and rigorous, requiring extensive validation studies to demonstrate clinical utility and safety, which can slow down market entry and commercialization. The lack of comprehensive clinical databases and established diagnostic algorithms for many microRNA signatures also presents a challenge for routine clinical use. Moreover, the interpretation of microRNA expression profiles requires specialized bioinformatics expertise, which may not be readily available in all clinical laboratories. Finally, the ongoing need for continuous research and development to discover new biomarkers and refine existing detection technologies demands significant and sustained investment, which can be a restraint for smaller market players.
Dominant Segments and Regions: RT-PCR Detection Kits and North America
The RT-PCR Detection Kit segment is projected to be a dominant force within the global microRNA detection kit market. This dominance stems from the technology's established reliability, high sensitivity, specificity, and cost-effectiveness for quantitative analysis of microRNA expression. RT-PCR (Reverse Transcription Polymerase Chain Reaction) kits are widely adopted due to their ability to accurately measure microRNA levels from various biological samples, including tissues, cells, and bodily fluids. The streamlined workflow and relative ease of use compared to more complex high-throughput methods contribute to their widespread application in both research and clinical settings. The ability to perform multiplexed assays, detecting multiple microRNAs simultaneously, further enhances their appeal. The market for RT-PCR detection kits is further bolstered by the continuous innovation in primer and probe design, as well as the development of advanced instrumentation that offers faster turnaround times and improved data analysis capabilities. The ongoing research into microRNA biomarkers for a vast array of diseases, from oncology to infectious diseases, directly translates into a sustained demand for robust and quantitative RT-PCR detection solutions. Companies like Thermo Fisher Scientific and Abcam are at the forefront of developing and marketing comprehensive RT-PCR kits that cater to diverse research and diagnostic needs. The versatility of RT-PCR also allows for its integration into various diagnostic workflows, making it a cornerstone technology for microRNA profiling.
North America is anticipated to emerge as a leading region in the global microRNA detection kit market. This regional dominance is attributed to several synergistic factors, including a robust healthcare infrastructure, significant investment in life sciences research and development, and a high prevalence of chronic diseases, which drives the demand for advanced diagnostic tools. The United States, in particular, boasts a vibrant ecosystem of academic institutions, pharmaceutical companies, and biotechnology firms actively engaged in microRNA research and the development of novel diagnostic and therapeutic solutions. Government funding for biomedical research, coupled with substantial private sector investment, fuels innovation and the adoption of cutting-edge technologies. The presence of leading global players such as Thermo Fisher Scientific, Abcam, and Agilent, with their extensive product portfolios and strong market presence in North America, further solidifies the region's leadership. The increasing awareness among healthcare professionals and the general public regarding the potential of microRNAs in early disease detection and personalized medicine also contributes to market growth. Furthermore, the region has a well-established regulatory framework that, while stringent, facilitates the approval and commercialization of innovative diagnostic kits once they meet the required standards. The adoption of liquid biopsy technologies for cancer screening and monitoring is particularly advanced in North America, directly benefiting the microRNA detection kit market. The continuous pipeline of clinical trials and research studies focused on microRNA biomarkers within North America ensures a sustained demand for high-quality detection kits.
The microRNA detection kit industry is propelled by several key growth catalysts. The intensifying focus on early disease detection and the increasing prevalence of chronic conditions like cancer and cardiovascular diseases are primary drivers. MicroRNAs are increasingly recognized as sensitive biomarkers for these diseases, creating a substantial demand for reliable detection kits. The burgeoning field of liquid biopsy, enabling non-invasive sample analysis, further fuels market expansion as it offers a more patient-friendly diagnostic approach. Advancements in molecular biology techniques, leading to more sensitive, specific, and rapid detection methods like RT-PCR, also play a crucial role. Finally, significant investments in life sciences research and development are continuously uncovering new microRNA targets and applications, thereby expanding the market's scope and potential.
This comprehensive report provides an in-depth analysis of the global microRNA detection kit market, encompassing a detailed examination of market trends, driving forces, and significant challenges. It offers granular insights into key regional and segment dominance, with a particular focus on the growth of RT-PCR detection kits and the leadership of North America. The report meticulously outlines the growth catalysts propelling the industry forward, such as the demand for early disease detection, the rise of liquid biopsy, and technological advancements. It further presents a detailed list of leading market players and chronicles significant developments, including product launches and strategic partnerships, from 2019 to 2023. With a study period extending to 2033 and a base year of 2025, the report provides robust forecasts and valuable intelligence for stakeholders aiming to navigate and capitalize on the dynamic microRNA detection kit landscape.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Thermo Fisher Scientific, Abcam, Agilent, BioTNT, MicroMedMark, Jusbio Sciences, Shenzhen Genebiohealth, BioDynamics Laboratory, MiRXES, Shanghai Dunwill, .
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "MicroRNA Detection Kit," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the MicroRNA Detection Kit, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.