1. What is the projected Compound Annual Growth Rate (CAGR) of the Instant Asthma Relief Device?
The projected CAGR is approximately 1.9%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Instant Asthma Relief Device
Instant Asthma Relief DeviceInstant Asthma Relief Device by Application (Hospital, Clinics, Others, World Instant Asthma Relief Device Production ), by Type (Metered-Dose Inhaler, Dry-Powder Inhaler, Others, World Instant Asthma Relief Device Production ), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The global Instant Asthma Relief Device market is poised for steady growth, projected to reach approximately USD 27 billion by 2025. This represents a compound annual growth rate (CAGR) of 1.9% from 2019 to 2033, indicating a maturing yet consistently in-demand market. The primary drivers fueling this expansion are the increasing prevalence of asthma globally, driven by factors such as air pollution, changing lifestyles, and improved diagnostic capabilities. Furthermore, the growing awareness among patients and healthcare providers about the importance of immediate symptom relief and effective asthma management strategies contributes significantly to market demand. Technological advancements leading to the development of more portable, user-friendly, and efficient inhaler devices, such as smart inhalers and breath-actuated nebulizers, are also key trends shaping the market landscape.
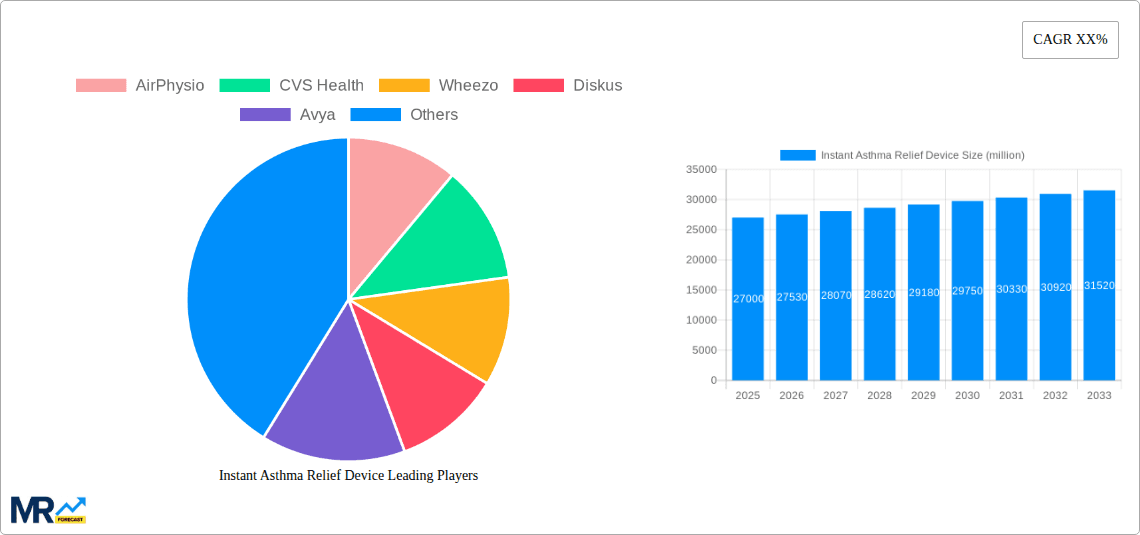

The market is segmented by application into hospitals, clinics, and others, with hospitals and clinics expected to be the dominant segments due to higher patient volume and established treatment protocols. By type, the market includes Metered-Dose Inhalers (MDIs), Dry-Powder Inhalers (DPIs), and others. MDIs and DPIs are likely to maintain their leading positions due to their widespread adoption and proven efficacy. However, challenges such as the high cost of advanced devices, potential side effects of some medications, and the availability of alternative treatment options can act as restraints. Geographically, North America and Europe are expected to be significant markets due to advanced healthcare infrastructure and high disposable incomes. The Asia Pacific region, however, is anticipated to witness the highest growth rate owing to a rising patient base and increasing healthcare expenditure. Key players like AirPhysio, CVS Health, and Wheezo are focusing on product innovation and strategic partnerships to capitalize on these evolving market dynamics.


Here's a unique report description for an "Instant Asthma Relief Device" market study, incorporating your specified elements:
This comprehensive report delves into the intricate dynamics of the global Instant Asthma Relief Device market, offering an in-depth analysis from the historical period of 2019-2024 to the projected forecast period of 2025-2033. With a base year of 2025, the study meticulously examines production, consumption, and market trends, anticipating a market value that will soon be measured in the tens of billions of US dollars. Our robust research methodology employs quantitative and qualitative analysis to provide actionable insights into market segmentation, key drivers, prevailing challenges, and the strategic moves of leading industry players. The report is an indispensable resource for stakeholders seeking to understand the evolving landscape, capitalize on emerging opportunities, and navigate the complexities of this vital healthcare sector.
The global Instant Asthma Relief Device market is poised for significant expansion, driven by a confluence of factors including the escalating prevalence of respiratory illnesses and a growing demand for rapid, portable, and effective symptom management solutions. XXX, a key indicator of market sentiment, suggests a robust CAGR that will see the market value surge into the tens of billions of US dollars by 2033. This growth is not merely quantitative; it reflects a qualitative shift in how asthma is managed, moving towards more patient-centric and accessible devices. The historical period (2019-2024) has witnessed incremental technological advancements, particularly in the miniaturization and improved efficacy of devices like Metered-Dose Inhalers (MDIs) and Dry-Powder Inhalers (DPIs). However, the estimated year of 2025 marks a pivotal point, with an anticipated acceleration in innovation and market penetration. The focus is increasingly shifting towards smart devices that offer data tracking and personalized treatment, bridging the gap between traditional relief and proactive respiratory care. Furthermore, the increasing awareness among patients and healthcare providers regarding the importance of timely relief to prevent severe asthma exacerbations is a crucial underpinning of this upward trajectory. The demand for devices that are easy to use, require minimal maintenance, and can be carried conveniently by individuals, regardless of their location or access to medical facilities, is a defining characteristic of the current market. This trend is further amplified by the rising disposable incomes in emerging economies, enabling a larger segment of the population to access advanced healthcare solutions. The market is also seeing a greater emphasis on user-friendly interfaces and discreet designs, addressing potential stigma associated with asthma management. The growing concern over air pollution and its impact on respiratory health globally is also a significant underlying factor, contributing to a sustained demand for effective asthma relief solutions. The integration of digital health technologies, such as smartphone connectivity and AI-powered diagnostics, is also on the horizon, promising to revolutionize how asthma is monitored and managed, further fueling the market's growth.
The burgeoning global Instant Asthma Relief Device market is being propelled by a powerful combination of rising asthma and COPD prevalence, increasing healthcare expenditure, and a growing emphasis on portable and user-friendly medical devices. The World Health Organization consistently reports a significant global burden of asthma, with millions of individuals worldwide grappling with this chronic respiratory condition. This escalating number of patients inherently translates to a higher demand for effective and immediate relief mechanisms. Furthermore, governments and healthcare organizations worldwide are increasing their investments in respiratory healthcare, recognizing the long-term economic and societal benefits of better asthma management. This includes funding for research and development of new and improved devices, as well as initiatives aimed at improving access to essential medications and equipment. The technological evolution of inhaler devices, moving from complex to simpler, more efficient, and discreet designs, is another critical driver. Patients are actively seeking solutions that offer convenience and empower them to manage their condition independently, reducing reliance on hospital visits. The convenience of carrying a compact and easy-to-operate device for immediate symptom relief in emergency situations is a significant factor influencing consumer choices. This is further supported by a growing health-conscious population that is more proactive in managing their well-being and seeking solutions that offer quick and effective results. The accessibility of these devices, through both pharmacies and increasingly, online platforms, also contributes to their widespread adoption.
Despite the promising growth trajectory, the Instant Asthma Relief Device market is not without its hurdles. One of the primary challenges is the high cost of advanced and innovative devices, which can limit accessibility for a significant portion of the population, particularly in low-income countries. This price sensitivity acts as a restraint on market penetration, even where demand is high. Another significant challenge is the need for proper patient education and adherence. Incorrect usage of inhaler devices can lead to reduced efficacy and potentially adverse outcomes, necessitating ongoing educational efforts from healthcare providers and manufacturers. The regulatory landscape, while crucial for ensuring product safety and efficacy, can also be a source of complexity and delay in bringing new devices to market. Navigating varying regulatory requirements across different regions adds to the development and approval timelines, impacting market entry strategies. Furthermore, the availability of generic alternatives and price competition from established players can put pressure on profit margins for manufacturers of newer, potentially more advanced devices. The risk of counterfeit products entering the market also poses a threat to both patient safety and brand reputation, requiring robust quality control and supply chain management. Finally, while technology is a driver, the rapid pace of technological obsolescence necessitates continuous investment in research and development to remain competitive, which can be a substantial financial burden for smaller companies.
The global Instant Asthma Relief Device market is projected to witness significant dominance by specific regions and segments, driven by a complex interplay of demographic trends, healthcare infrastructure, and economic factors.
North America is anticipated to emerge as a leading region, primarily due to:
Within the Application segment, the Hospital segment is expected to be a dominant force in the Instant Asthma Relief Device market.
In terms of Type, Metered-Dose Inhalers (MDIs) are likely to maintain a significant market share, though the growth of Dry-Powder Inhalers (DPIs) is also noteworthy.
The interplay between these regions and segments underscores the multifaceted nature of the Instant Asthma Relief Device market. While North America's advanced infrastructure and high prevalence create a strong demand, the application in hospitals for acute care and the established dominance of MDIs, coupled with the growing adoption of DPIs, highlight the diverse pathways to market leadership. The forecast period (2025-2033) is expected to see continued evolution in these dynamics as new technologies emerge and healthcare access expands globally.
Several key catalysts are poised to significantly propel the Instant Asthma Relief Device industry forward. The escalating global burden of respiratory diseases, particularly asthma and COPD, is a primary driver, creating a persistent and growing demand for effective symptom management. Technological advancements, leading to more portable, user-friendly, and data-enabled devices, are enhancing patient adherence and treatment efficacy. Furthermore, increasing healthcare expenditure and government initiatives focused on improving respiratory health access in both developed and emerging economies will foster market expansion. The growing health consciousness among individuals and their proactive approach to managing chronic conditions also contribute to this positive outlook.
This report offers unparalleled comprehensive coverage of the Instant Asthma Relief Device market, providing a meticulous analysis of its current state and future trajectory. It meticulously dissects the market into key segments, including applications like Hospitals and Clinics, alongside broader "Others" categories, and different device types such as Metered-Dose Inhalers, Dry-Powder Inhalers, and a comprehensive "Others" classification. By examining the World Instant Asthma Relief Device Production across these segments, the report paints a detailed picture of global supply and demand dynamics. The historical analysis from 2019-2024, a detailed outlook for the base and estimated year of 2025, and a robust forecast for 2025-2033, ensures that stakeholders have access to actionable intelligence for strategic planning. The report goes beyond mere data, offering in-depth insights into industry developments, growth catalysts, and the competitive landscape populated by leading players.


| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 1.9% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 1.9%.
Key companies in the market include AirPhysio, CVS Health, Wheezo, Diskus, Avya.
The market segments include Application, Type.
The market size is estimated to be USD XXX N/A as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in N/A and volume, measured in K.
Yes, the market keyword associated with the report is "Instant Asthma Relief Device," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Instant Asthma Relief Device, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.