1. What is the projected Compound Annual Growth Rate (CAGR) of the Human Vaccine?
The projected CAGR is approximately 4.4%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Human Vaccine
Human VaccineHuman Vaccine by Type (Varicella, Influenza, Polio, Hepatitis A, Rabies, BCG, Hepatitis B, Pertussis, Diphtheria, Tetanus, Pneumococcal, Rota vaccine), by Application (Adults, Children), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
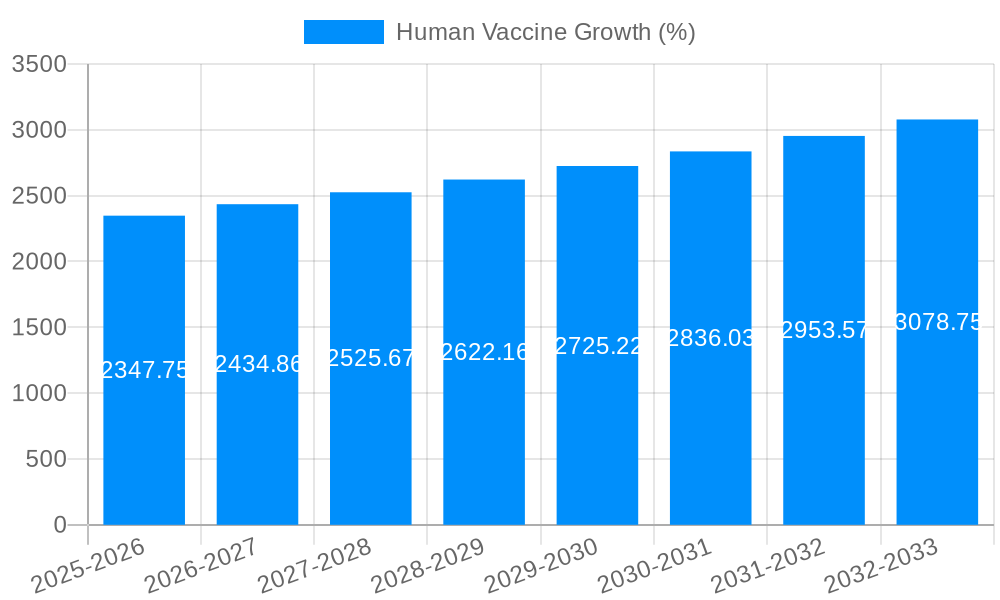
The global human vaccine market is poised for robust growth, projected to reach a substantial USD 32,870 million by 2025. Driven by increasing awareness of infectious disease prevention, rising healthcare expenditures, and the continuous development of novel vaccines, the market is expected to expand at a Compound Annual Growth Rate (CAGR) of 4.4% through 2033. Key growth drivers include the growing burden of chronic and infectious diseases, such as influenza, hepatitis, and pneumococcal infections, which necessitate widespread vaccination programs. Furthermore, government initiatives promoting immunization, coupled with advancements in vaccine technology like mRNA platforms, are significantly contributing to market expansion. The pediatric segment, encompassing vaccines for varicella, influenza, polio, hepatitis A, hepatitis B, pertussis, diphtheria, tetanus, pneumococcal, and rotavirus, will continue to dominate the market due to routine immunization schedules. However, the adult vaccination segment is witnessing a surge in demand, fueled by an aging global population susceptible to various infections and the increasing availability of vaccines for conditions like shingles and pneumococcal disease in older adults.
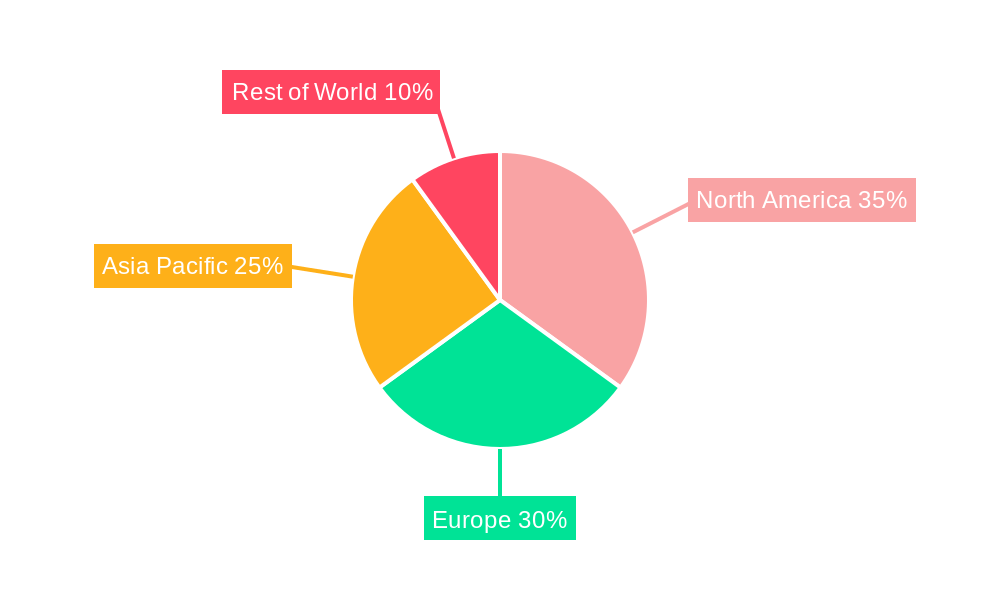
The market landscape is characterized by intense competition among leading pharmaceutical and biotechnology companies, including established players like GSK, SANOFI, SINOVAC BIOTECH, and emerging players like Bharat Biotech and Walvax Biotechnology. Strategic collaborations, mergers, and acquisitions are common strategies employed by these companies to expand their product portfolios and geographical reach. Geographically, the Asia Pacific region is emerging as a significant growth engine, attributed to a large and growing population, increasing disposable incomes, and government investments in public health infrastructure. North America and Europe remain mature yet substantial markets, driven by advanced healthcare systems and strong regulatory frameworks. Restrains to market growth, such as vaccine hesitancy, stringent regulatory approvals, and the high cost of research and development, are being addressed through public awareness campaigns and technological innovations aimed at reducing production costs and improving vaccine efficacy. The forecast period will likely see a sustained focus on developing vaccines for emerging infectious diseases and enhancing existing vaccine formulations.
XXX, the global human vaccine market is poised for significant expansion, driven by increasing awareness of preventative healthcare, rising infectious disease outbreaks, and advancements in vaccine technology. The market is projected to reach substantial figures in terms of unit sales, with projections indicating a surge from approximately 850 million units in the Historical Period (2019-2024) to an estimated 1,500 million units by the Base Year (2025) and further escalating to over 2,200 million units by the end of the Forecast Period (2033). This robust growth is underpinned by several key trends. A primary driver is the continued focus on routine immunization programs, particularly for childhood diseases like Polio, Diphtheria, Tetanus, Pertussis, and Hepatitis B, which remain critical for global public health. The increasing prevalence of chronic conditions and the aging global population are also fueling demand for vaccines against diseases like Influenza and Pneumococcal infections. Furthermore, the development and rapid deployment of novel vaccines, exemplified by the response to recent global health crises, highlight the industry's adaptability and innovation. The expansion of vaccine accessibility in emerging economies, supported by government initiatives and partnerships, is another significant trend contributing to the market's upward trajectory. Companies are increasingly investing in research and development to create more effective and safer vaccines, including combination vaccines that offer protection against multiple diseases, thereby improving compliance and cost-effectiveness. The market dynamics are also being shaped by evolving regulatory landscapes and a growing emphasis on supply chain robustness to ensure equitable distribution. The proactive approach to disease prevention through vaccination is becoming a cornerstone of public health strategies worldwide, solidifying its position as a vital segment of the healthcare industry.
The human vaccine market is experiencing a remarkable upward trajectory, propelled by a confluence of potent driving forces. A paramount factor is the escalating global health consciousness, a trend amplified by recent pandemics that have underscored the critical role of vaccination in safeguarding populations. Governments and international health organizations are intensifying their efforts to promote vaccination through robust public health campaigns and expanded immunization programs. This heightened awareness translates directly into increased demand for a wide spectrum of vaccines. Furthermore, the persistent threat of infectious disease outbreaks, both endemic and emerging, necessitates continuous development and deployment of effective vaccines. The rapid evolution of pathogens also compels ongoing research into novel vaccine candidates and the enhancement of existing formulations. Technological advancements in vaccine development, including the adoption of mRNA platforms and novel adjuvant technologies, are accelerating the creation of more potent and targeted vaccines. These innovations not only improve efficacy but also pave the way for vaccines against previously untreatable or poorly managed diseases. The increasing investment in research and development by leading pharmaceutical and biotechnology companies, coupled with supportive government funding and favorable regulatory pathways for innovative vaccines, further fuels this growth. The expansion of healthcare infrastructure, particularly in developing regions, is also broadening access to vaccination services, thereby creating new market opportunities and driving unit sales.
Despite the optimistic outlook, the human vaccine market encounters several significant challenges and restraints that warrant careful consideration. A primary hurdle is the complex and often lengthy regulatory approval process for new vaccines, which can delay market entry and increase development costs. Stringent safety and efficacy requirements, while essential, can pose a significant barrier, especially for novel vaccine technologies. Public perception and vaccine hesitancy remain a persistent concern in many regions, fueled by misinformation and a lack of trust in scientific institutions. This can lead to lower vaccination rates and resistance to public health mandates, directly impacting market demand. Furthermore, the high cost of research, development, and manufacturing of vaccines, particularly for rare diseases or those requiring complex production processes, can limit accessibility, especially in low-income countries. Supply chain disruptions, exacerbated by geopolitical events, natural disasters, or manufacturing issues, can also create shortages and hinder the timely distribution of essential vaccines. Intellectual property disputes and the potential for patent cliffs can create uncertainties for market players. Additionally, the emergence of new infectious diseases requires rapid vaccine development and production, posing a challenge for preparedness and response. The economic burden of vaccine procurement on healthcare systems and governments can also be a limiting factor, particularly in resource-constrained environments, necessitating careful planning and allocation of funds.
The human vaccine market is characterized by dynamic regional and segment-specific dominance, with several key players and areas contributing significantly to overall growth.
Dominant Regions/Countries:
Dominant Segments:
The human vaccine industry is experiencing robust growth driven by several key catalysts. The increasing global awareness of the importance of preventative healthcare and the effectiveness of vaccines in curbing infectious diseases is a primary driver. Furthermore, the continuous threat of emerging and re-emerging infectious diseases necessitates ongoing vaccine development and deployment. Technological advancements in vaccine design, such as mRNA technology and novel adjuvant systems, are enabling the creation of more effective and faster-to-produce vaccines. Government initiatives and international health organizations' commitment to expanding immunization coverage, particularly in developing regions, are also significant growth engines.
This comprehensive report delves into the multifaceted human vaccine market, offering an in-depth analysis of its dynamics from 2019 to 2033. It provides crucial insights into market trends, future projections, and the intricate interplay of driving forces and challenges. The report meticulously examines key regions and segments poised for dominance, offering strategic perspectives on market penetration. Furthermore, it highlights the pivotal growth catalysts propelling the industry forward and identifies the leading players shaping its landscape. The report also chronicles significant historical and upcoming developments, offering a holistic view of the vaccine sector's evolution. This extensive coverage empowers stakeholders with the knowledge necessary to navigate this critical and ever-evolving healthcare domain.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 4.4% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 4.4%.
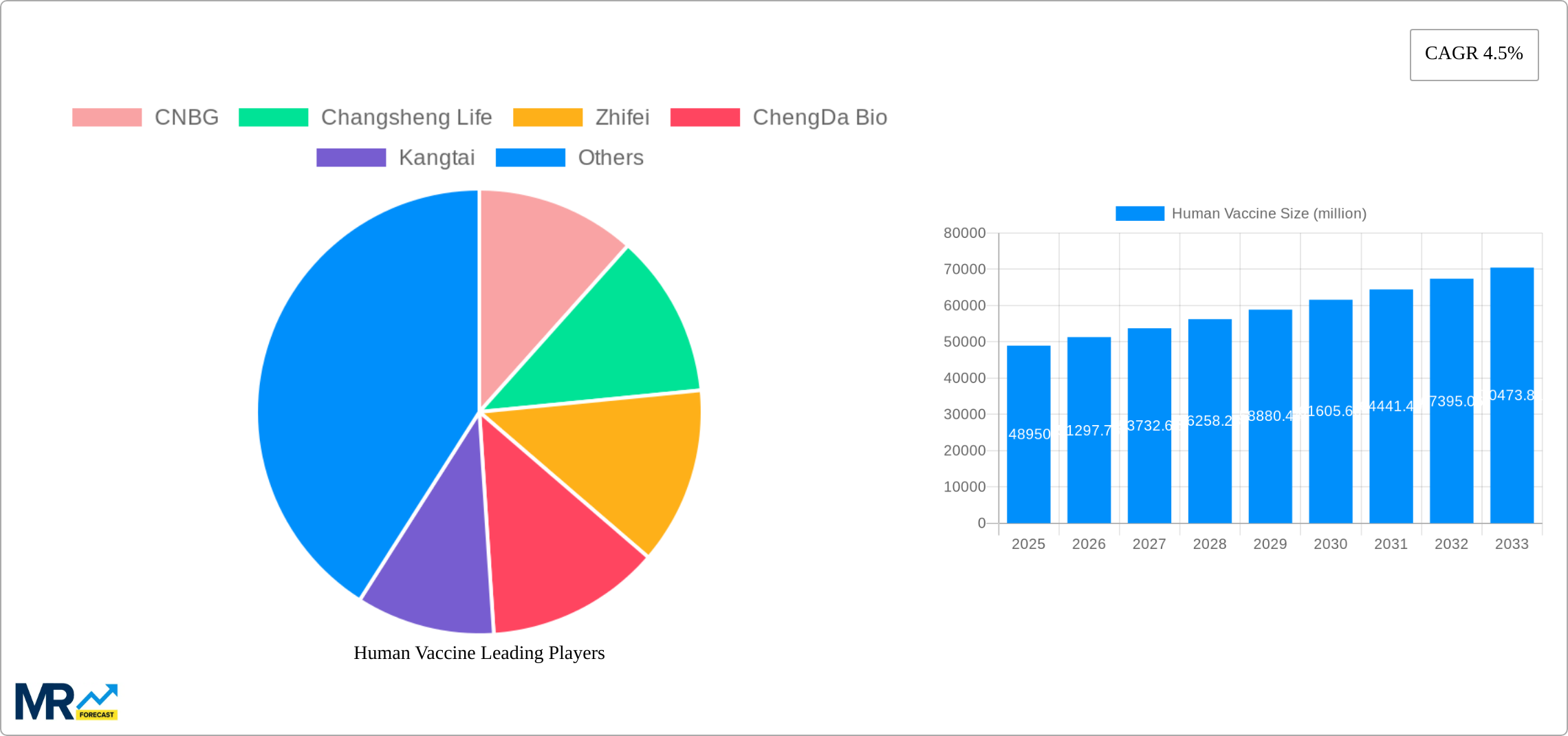
Key companies in the market include CNBG, Changsheng Life, Zhifei, ChengDa Bio, Kangtai, SINOVAC BIOTECH, Hissen, Walvax Biotechnology, GSK, SANOFI, Rong An, NuoCheng Bio, Hualan Bio, Tiantan biological, Changchun Baike, Adimmune, Zhongyianke Biotech, Bharat Biotech, Bavarian Nordic, Sanofi-Pasteur, Indian Immunologicals, KANGH, Henan Grand Biopharma, ZhongKe Biopharm, Zhuoyi Biological, .
The market segments include Type, Application.
The market size is estimated to be USD 32870 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Human Vaccine," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Human Vaccine, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.