1. What is the projected Compound Annual Growth Rate (CAGR) of the Home Nucleic Acid Testing Kit?
The projected CAGR is approximately 5%.
 Home Nucleic Acid Testing Kit
Home Nucleic Acid Testing KitHome Nucleic Acid Testing Kit by Application (Hospitals, Pathology Laboratories, Research Institutes, Clinics, Others), by Type (Transcription-mediated Amplification (TMA), Polymerase Chain Reaction (PCR), Ligase Chain Reaction (LCR), Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The home nucleic acid testing (NAT) kit market is experiencing robust growth, driven by increasing consumer awareness of at-home diagnostics, rising incidences of infectious diseases, and the convenience of self-testing. The market's 5% CAGR, while seemingly modest, represents significant expansion considering the relatively recent emergence of this technology. This growth is further fueled by technological advancements leading to more accurate, user-friendly, and affordable NAT kits. The market is segmented by test type (e.g., COVID-19, influenza, sexually transmitted infections), technology (e.g., PCR, LAMP), and distribution channels (e.g., online retailers, pharmacies). Key players, including Agilent Technologies, Danaher Corporation, and Abbott Laboratories, are investing heavily in R&D and strategic partnerships to expand their market share and product offerings. The market's accessibility is also growing as regulatory hurdles are overcome and reimbursement policies evolve, fostering greater adoption.

Despite the considerable market potential, several challenges persist. These include concerns about test accuracy, the potential for misinterpretation of results leading to self-medication or delayed professional medical attention, and the need for robust quality control measures. Furthermore, variations in regulatory frameworks across different regions pose significant obstacles to global market penetration. Nonetheless, the ongoing pandemic-driven demand for rapid and convenient diagnostic tools, combined with continuous technological innovation and increasing consumer acceptance, suggest a highly positive outlook for the home NAT kit market over the forecast period (2025-2033). The market is expected to see continued expansion, particularly in regions with well-developed healthcare infrastructure and high internet penetration. Future growth will heavily depend on consumer trust in at-home diagnostic technology, the development of more sophisticated and user-friendly kits, and continued expansion of product offerings beyond infectious disease testing.

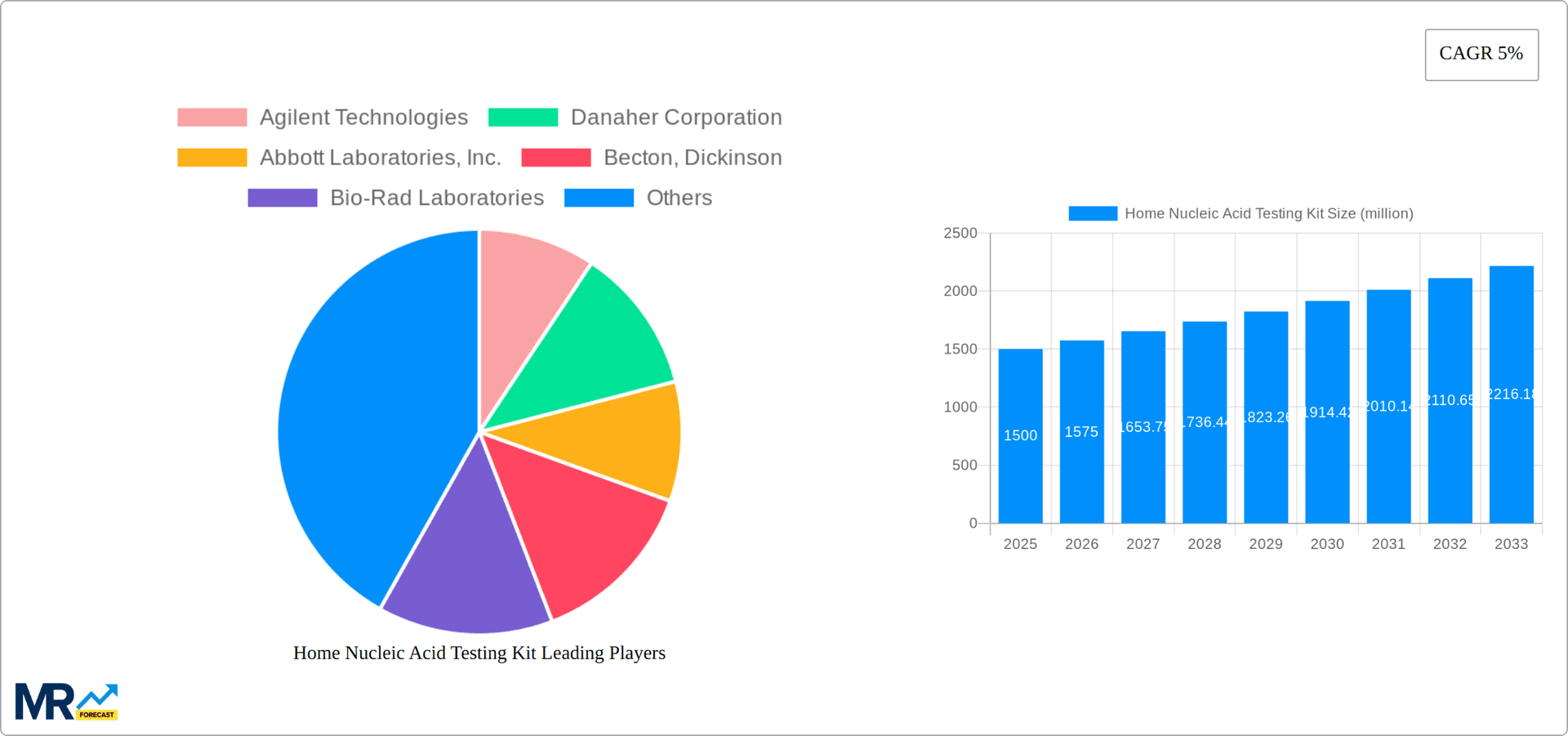
The home nucleic acid testing kit market is experiencing explosive growth, projected to reach multi-million unit sales by 2033. Driven by increasing consumer awareness of health and wellness, coupled with technological advancements making these tests more accessible and user-friendly, the market has seen significant expansion, especially post-2019. The historical period (2019-2024) witnessed a surge in demand fueled primarily by the COVID-19 pandemic, highlighting the potential for rapid adoption of at-home diagnostics. The base year of 2025 shows a market already exceeding several million units sold, indicating sustained growth even beyond the initial pandemic-driven boost. This growth is not solely reliant on infectious disease testing; the market is diversifying into areas like genetic testing, enabling consumers to proactively manage their health and wellness. This shift toward proactive healthcare is a key driver, as consumers seek convenient and cost-effective ways to monitor their health parameters without the need for extensive medical appointments. The forecast period (2025-2033) promises continued expansion, particularly in regions with robust healthcare infrastructure and higher disposable incomes. The market is also experiencing a maturation process with improved accuracy, decreased testing times, and simpler user interfaces contributing to greater consumer acceptance. However, regulatory hurdles and concerns about data privacy remain challenges that need to be addressed to fully unlock the market's potential. The evolving landscape of telehealth integration offers exciting opportunities, potentially streamlining the process further by allowing for remote consultation and result interpretation. In summary, the home nucleic acid testing kit market shows incredible promise, with a strong trajectory fueled by technological advancements, increasing consumer demand for personalized healthcare, and a constantly evolving regulatory environment.
Several key factors propel the growth of the home nucleic acid testing kit market. The rising prevalence of chronic diseases globally necessitates convenient and early diagnostic tools, making at-home testing a highly attractive option. Technological advancements, such as CRISPR technology and next-generation sequencing, are constantly improving the accuracy, speed, and affordability of these tests. Simultaneously, increasing consumer awareness of preventative healthcare and personalized medicine contributes to a heightened demand for convenient self-testing options. The expanding e-commerce landscape provides a platform for easy access to these kits, further boosting market growth. Furthermore, government initiatives promoting point-of-care diagnostics and telehealth are creating a favorable regulatory environment for the adoption of home nucleic acid testing. The COVID-19 pandemic served as a powerful catalyst, demonstrating the effectiveness and convenience of rapid at-home testing, leading to a massive increase in both consumer and public health awareness. Finally, the continuous reduction in the cost of nucleic acid testing technologies makes them increasingly accessible to a wider population, further accelerating market expansion across various socioeconomic groups and geographical regions.
Despite its promising trajectory, the home nucleic acid testing kit market faces several challenges. Accuracy and reliability remain critical concerns, as inaccurate results can lead to delayed or inappropriate medical interventions. The complexity of some tests and the need for proper sample collection techniques can hinder widespread adoption, particularly amongst less tech-savvy individuals. Regulatory hurdles and varying standards across different countries create complexities for manufacturers seeking global market access. Data privacy and security also pose significant concerns, as these tests often involve the collection and storage of sensitive personal genetic information. Ensuring the confidentiality and integrity of this data is paramount for maintaining consumer trust. Furthermore, the potential for misuse and misinterpretation of results necessitates robust educational programs and clear instructions for consumers. Finally, the need for consistent quality control and assurance throughout the entire testing process, from sample collection to result interpretation, is crucial for maintaining the credibility and reliability of home nucleic acid testing. Addressing these challenges is crucial for unlocking the full potential of this rapidly evolving market.
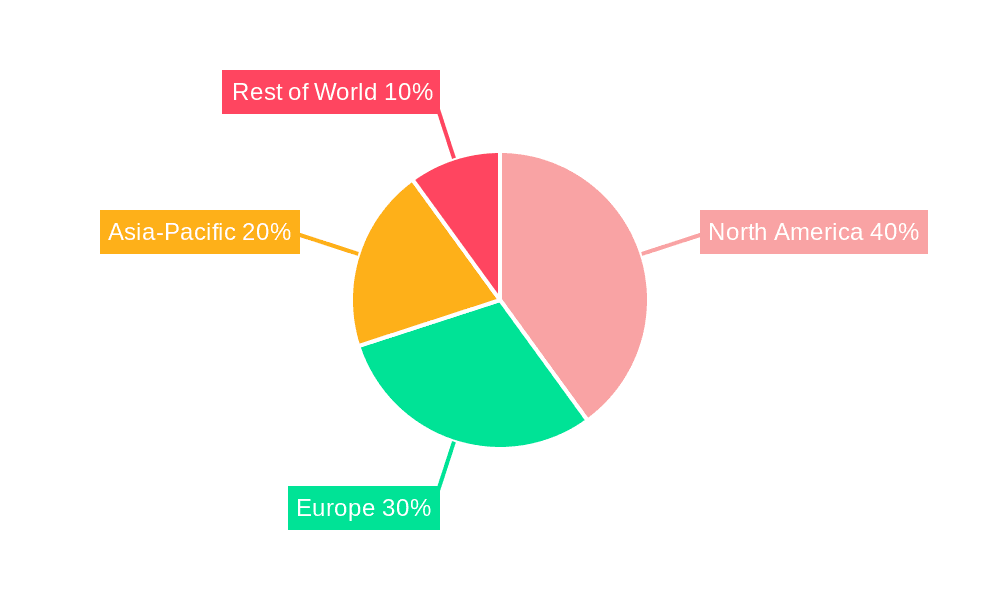
The North American and European markets are currently leading the home nucleic acid testing kit market due to factors like high disposable incomes, advanced healthcare infrastructure, and strong regulatory frameworks. However, the Asia-Pacific region is showing promising growth potential driven by increasing healthcare expenditure and rising awareness of health and wellness.
Segments: The segments driving the most growth are those focused on:
These segments contribute significantly to the overall market value and unit sales reaching into the millions. The diverse applications of home nucleic acid testing across various health concerns are fuelling growth and pushing total unit sales well into the millions.
Several factors are catalyzing the growth of the home nucleic acid testing kit industry. These include the increasing prevalence of chronic diseases, technological advancements that improve accuracy and affordability, rising consumer awareness of preventative healthcare, and the expanding e-commerce landscape facilitating easy access to these tests. Government initiatives promoting telehealth and point-of-care diagnostics also play a vital role. Finally, the COVID-19 pandemic demonstrated the value and convenience of at-home testing, significantly boosting market awareness and adoption rates. These factors collectively contribute to the rapid expansion of this dynamic market.
This report provides a comprehensive analysis of the home nucleic acid testing kit market, covering market size, trends, drivers, challenges, key players, and future growth projections. It offers valuable insights for companies operating in this sector, as well as investors seeking to understand the market dynamics and opportunities. The report's detailed segmentation and regional analysis provide a granular understanding of the market, enabling informed strategic decision-making. The report also includes forecasts for the next decade, offering a long-term perspective on the market's potential.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 5%.
Key companies in the market include Agilent Technologies, Danaher Corporation, Abbott Laboratories, Inc., Becton, Dickinson, Bio-Rad Laboratories, Roche, Illumina, Inc, Thermo Fisher Scientific Inc., Merck KGaA, Qiagen, .
The market segments include Application, Type.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Home Nucleic Acid Testing Kit," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Home Nucleic Acid Testing Kit, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.