1. What is the projected Compound Annual Growth Rate (CAGR) of the Hepatitis E virus(HEV) Rapid Test Kit?
The projected CAGR is approximately 6.45%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Hepatitis E virus(HEV) Rapid Test Kit
Hepatitis E virus(HEV) Rapid Test KitHepatitis E virus(HEV) Rapid Test Kit by Type (Enzyme-Linked Immunoassay, Probe Method, Colloidal Gold Method, Others, World Hepatitis E virus(HEV) Rapid Test Kit Production ), by Application (Diagnostic Center, Hospital, Family Expenses, World Hepatitis E virus(HEV) Rapid Test Kit Production ), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
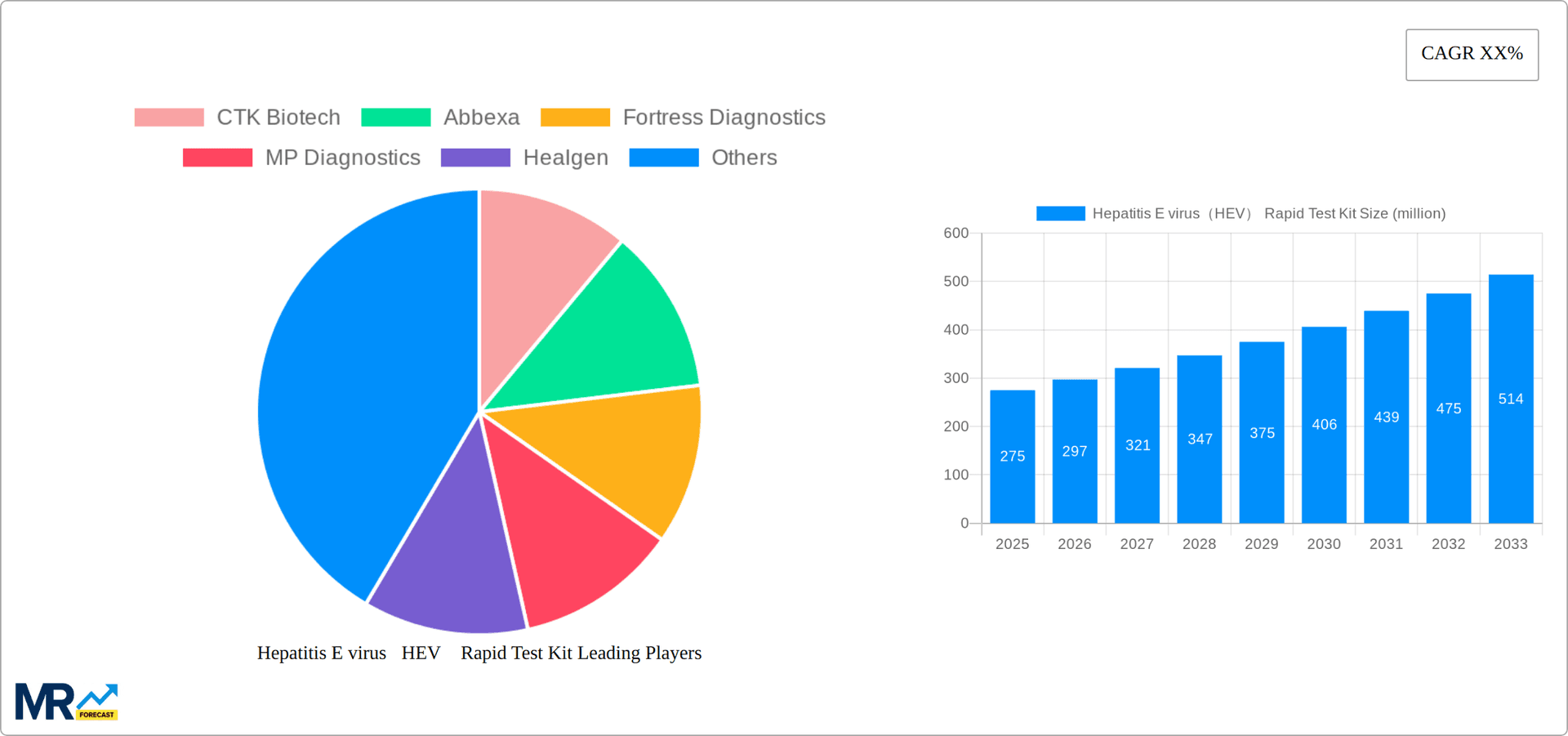
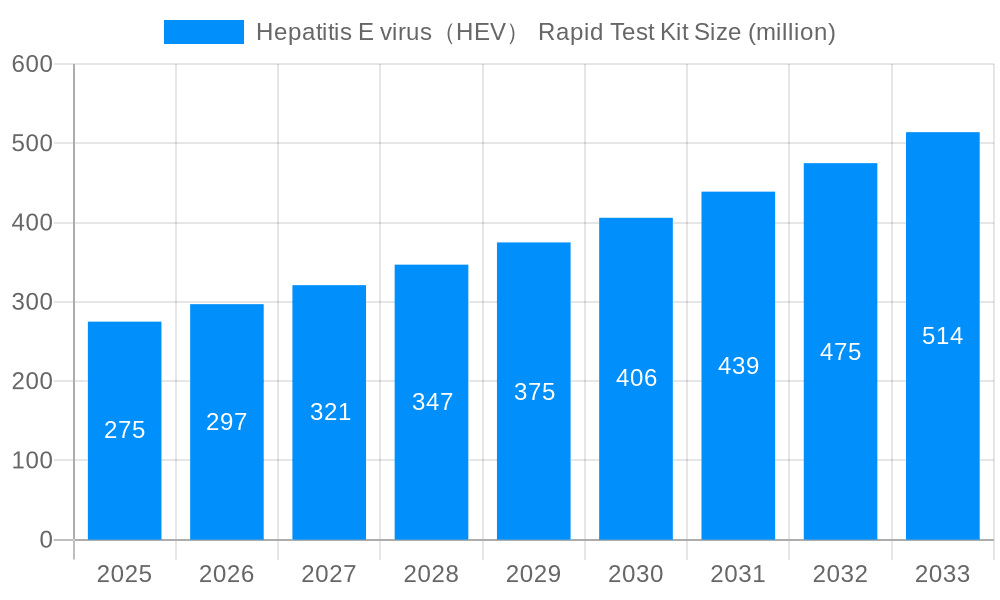
The Hepatitis E Virus (HEV) Rapid Test Kit market is projected for substantial growth, driven by increasing global HEV infection rates, rising demand for rapid point-of-care diagnostics, and advancements in test technology enhancing accuracy and usability. Government initiatives promoting sanitation and hygiene, alongside targeted awareness campaigns for at-risk populations, are also key market drivers. The market size is estimated at 1520.75 million in the base year 2024, with a projected Compound Annual Growth Rate (CAGR) of 6.45%.

Key market segments include point-of-care kits, laboratory tests, and various formats like immunochromatographic assays and ELISA. Regional consumption will vary, with developed nations exhibiting higher per capita uptake due to greater healthcare access. Market challenges include HEV's comparatively lower prevalence versus other hepatitis types, potential accuracy issues in resource-limited settings, and competition from established diagnostic methods.

The competitive arena comprises both established diagnostic firms and emerging players, offering opportunities for consolidation and innovation. Companies with strong distribution networks and a proven history in infectious disease diagnostics are poised for significant roles. Mergers and acquisitions are anticipated to expand product portfolios and geographic reach. Technological advancements, particularly in sensitive and specific HEV rapid tests capable of detecting diverse genotypes, will be critical for future growth and improved patient outcomes. Continued research and development focused on affordability and accessibility in low-resource settings is essential for widespread market penetration and effective HEV infection control.
The global Hepatitis E virus (HEV) Rapid Test Kit market is experiencing robust growth, projected to reach several billion units by 2033. Driven by increasing HEV prevalence, particularly in developing nations with inadequate sanitation and hygiene, the demand for rapid, point-of-care diagnostic tools is surging. The market witnessed significant expansion during the historical period (2019-2024), fueled by technological advancements leading to more sensitive and user-friendly tests. The estimated market value in 2025 is expected to surpass several hundred million units, showcasing the industry's sustained momentum. This growth trajectory is further bolstered by government initiatives promoting disease surveillance and control programs, coupled with rising awareness among healthcare professionals and the general population regarding HEV infection. The forecast period (2025-2033) anticipates consistent growth, driven by factors such as increasing diagnostic testing rates, expansion of healthcare infrastructure, and the development of improved rapid diagnostic tests with higher accuracy and cost-effectiveness. This positive trend is likely to continue, with the market driven by the need for timely and efficient HEV diagnosis to enable prompt treatment and prevent disease transmission. The market's expansion also reflects a shift toward decentralized testing, enabling quicker diagnosis and treatment in remote areas. The increasing adoption of these tests in both developed and developing nations fuels the anticipated multi-billion unit market size by 2033.
Several factors contribute to the burgeoning Hepatitis E virus (HEV) Rapid Test Kit market. Firstly, the rising prevalence of HEV infections globally, particularly in regions with poor sanitation and hygiene, necessitates rapid and accessible diagnostic tools. Secondly, advancements in diagnostic technology have resulted in more sensitive, specific, and user-friendly rapid tests, making them attractive for both healthcare professionals and consumers. The increasing demand for point-of-care testing, enabling quicker diagnosis and treatment, particularly in resource-limited settings, further accelerates market growth. Government initiatives promoting disease surveillance and control programs, along with increased investment in healthcare infrastructure in developing countries, are also significant driving forces. Furthermore, growing awareness among healthcare professionals and the general public regarding HEV infection, its potential severity, and the importance of early diagnosis has significantly increased the demand for rapid diagnostic tests. The convenience and speed offered by these kits, reducing turnaround times for test results compared to traditional methods, represent another key driver of market expansion. Finally, the rising adoption of these kits in various settings, including hospitals, clinics, and even home-testing scenarios, continues to fuel the overall market growth.
Despite its robust growth, the Hepatitis E virus (HEV) Rapid Test Kit market faces certain challenges. One significant hurdle is the relatively high cost of some rapid tests, particularly in resource-limited settings, potentially limiting their accessibility to those who need them most. The accuracy and reliability of some rapid tests can also be variable, impacting diagnostic confidence and potentially leading to misdiagnosis or delayed treatment. The lack of awareness and understanding of HEV infection among some populations and healthcare providers can also hinder market expansion. Regulatory hurdles and varying approval processes in different countries might create complexities for manufacturers seeking to introduce their products into new markets. Furthermore, competition from established diagnostic testing methods, such as enzyme-linked immunosorbent assays (ELISAs), presents a challenge, although rapid tests are steadily gaining traction due to their convenience and speed. The potential for false-positive or false-negative results, though minimized with technological advancements, remains a concern that requires continuous improvement in test sensitivity and specificity. Finally, ensuring consistent quality control throughout the manufacturing process is crucial to maintain the reliability and market acceptance of HEV rapid test kits.
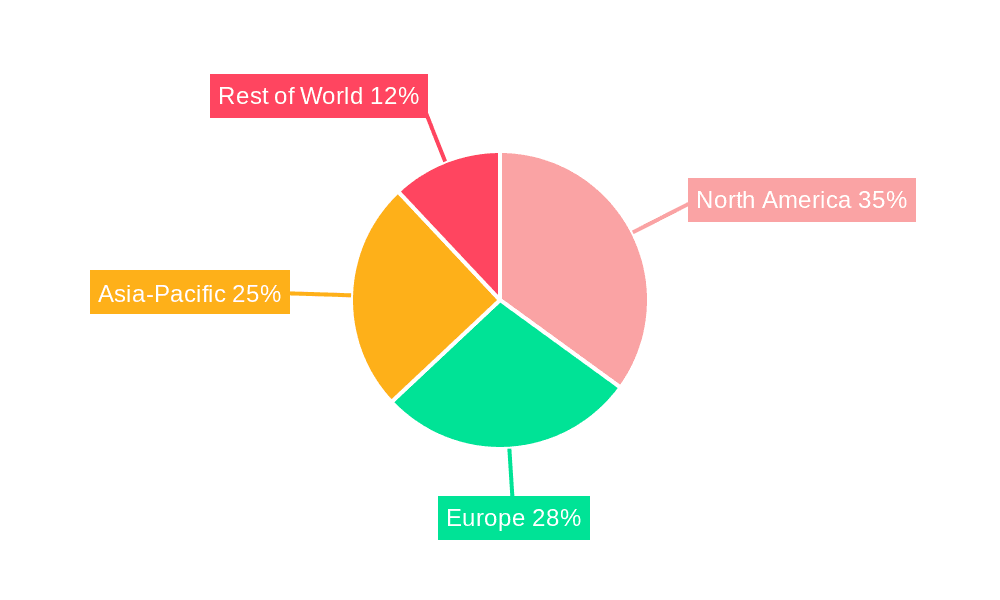
The Asia-Pacific region is expected to dominate the Hepatitis E virus (HEV) Rapid Test Kit market due to several factors:
Other regions, including Africa and parts of South America, are also experiencing substantial growth, albeit at a slower rate. The growth is driven by rising prevalence in these regions and initiatives to improve sanitation and access to healthcare.
The HEV rapid test kit industry is experiencing significant growth due to several key factors. Technological advancements are leading to more accurate, sensitive, and user-friendly tests. Increasing awareness of HEV among healthcare professionals and the public is driving demand for rapid diagnostic tools. Government initiatives promoting disease surveillance and control programs also contribute significantly. Finally, the rising adoption of point-of-care testing is making early and efficient diagnosis accessible, particularly in resource-limited settings, and significantly driving up the market's growth potential.
The report offers a comprehensive overview of the Hepatitis E virus (HEV) Rapid Test Kit market, analyzing historical data, present trends, and future projections. It provides detailed insights into market dynamics, driving forces, challenges, key players, and significant developments, offering valuable information for stakeholders across the industry. The report's extensive coverage helps readers understand market opportunities and potential challenges, supporting informed decision-making and strategic planning within the rapidly evolving landscape of HEV diagnostics.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6.45% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 6.45%.
Key companies in the market include CTK Biotech, Abbexa, Fortress Diagnostics, MP Diagnostics, Healgen, Bio-Medical Laboratory Supplies, Creative Diagnostics, Athenese-Dx, Biozek medical, Diagnostic Automation / Cortez Diagnostics, Zhejiang Orient Gene Biotech, Beijing Wantai Biological Pharmacy Enterprise, Lujia Botai Technology(Beijing), InTec, Xiamen Boson Biotech, Shanghai Rongsheng Biotech, ACON Laboratories, Shandong Meizheng Bio-Tech.
The market segments include Type, Application.
The market size is estimated to be USD 1520.75 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Hepatitis E virus(HEV) Rapid Test Kit," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Hepatitis E virus(HEV) Rapid Test Kit, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.