1. What is the projected Compound Annual Growth Rate (CAGR) of the Fecal Microbiota Transplantation?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Fecal Microbiota Transplantation
Fecal Microbiota TransplantationFecal Microbiota Transplantation by Type (/> Phase-I, Phase-2, Phase-3, Phase-4), by Application (/> Clostridium Difficile Infections, Parkinson Disease, Obesity, Diabetes Mellitus, Autism, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
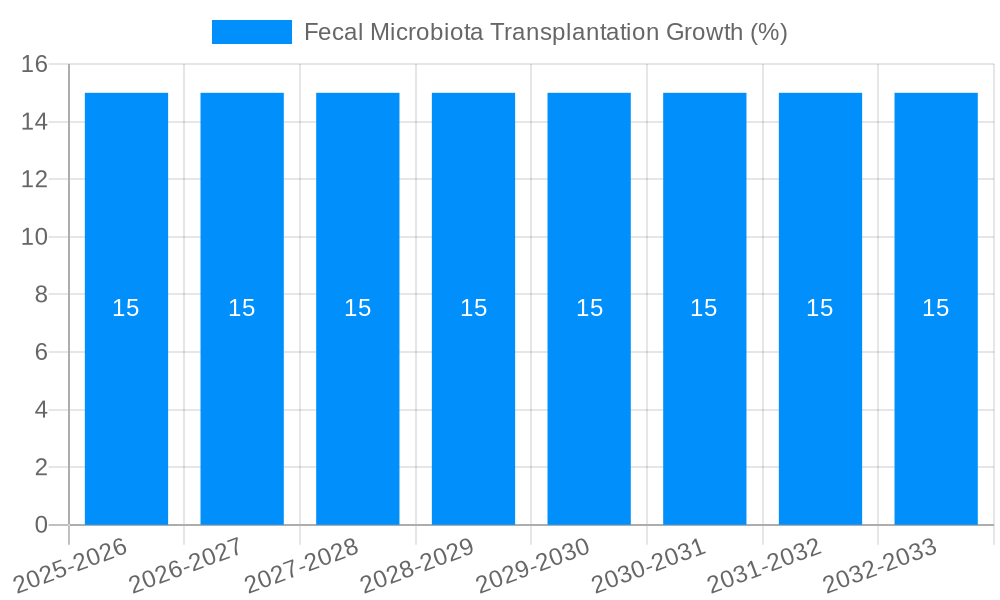
The global Fecal Microbiota Transplantation (FMT) market is poised for significant expansion, estimated at USD 392.4 million in 2025, with projections indicating a robust Compound Annual Growth Rate (CAGR) of approximately 15% over the forecast period of 2025-2033. This substantial growth is primarily driven by the increasing prevalence of chronic gastrointestinal disorders, particularly recurrent Clostridium difficile infections (CDI), where FMT has demonstrated remarkable efficacy. The expanding research and clinical applications for other conditions such as Parkinson's disease, obesity, diabetes mellitus, and autism spectrum disorder are also contributing to market momentum. Advances in donor screening, standardization of procedures, and the development of novel delivery methods are further fueling adoption and accessibility, making FMT a more viable therapeutic option for a wider patient base.
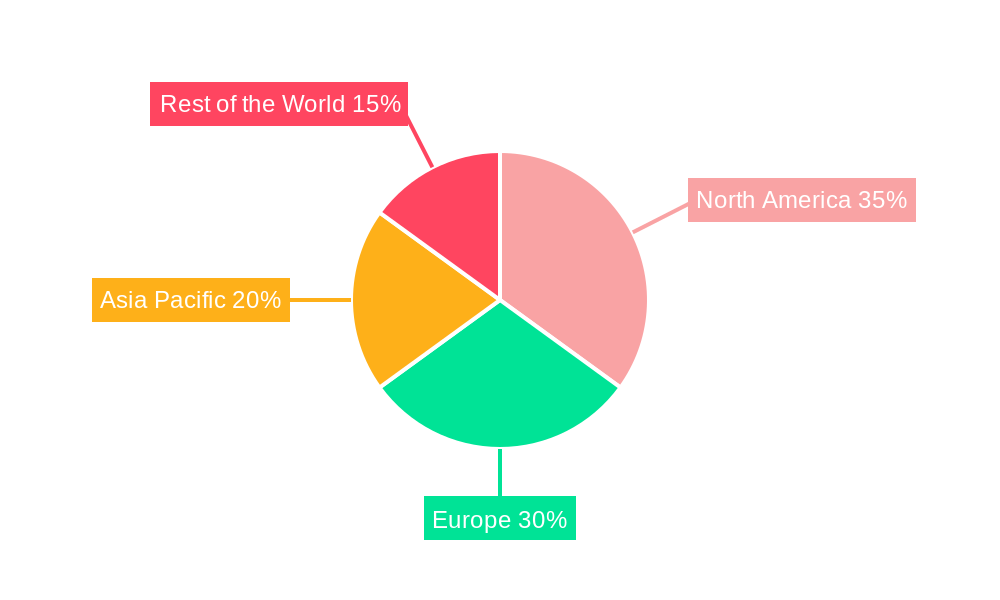
The market is segmented by phase, with Phase-3 trials representing a critical stage for many promising FMT-based therapies, indicating a strong pipeline for future market entrants. Applications in CDI remain the dominant segment, reflecting the established success and regulatory approvals for this indication. However, emerging applications in neurodegenerative diseases and metabolic disorders are anticipated to be key growth drivers in the long term. Geographically, North America and Europe currently lead the market, owing to advanced healthcare infrastructure, high research and development investments, and established regulatory pathways. The Asia Pacific region is expected to witness the fastest growth, driven by a large patient population, increasing healthcare expenditure, and a growing awareness of microbiome-based therapies. Restraints include the need for standardized regulatory frameworks across different regions, potential donor-related risks, and public perception challenges, though ongoing clinical trials and positive real-world data are steadily mitigating these concerns.
Here's a unique report description on Fecal Microbiota Transplantation, incorporating your specified elements and structure:
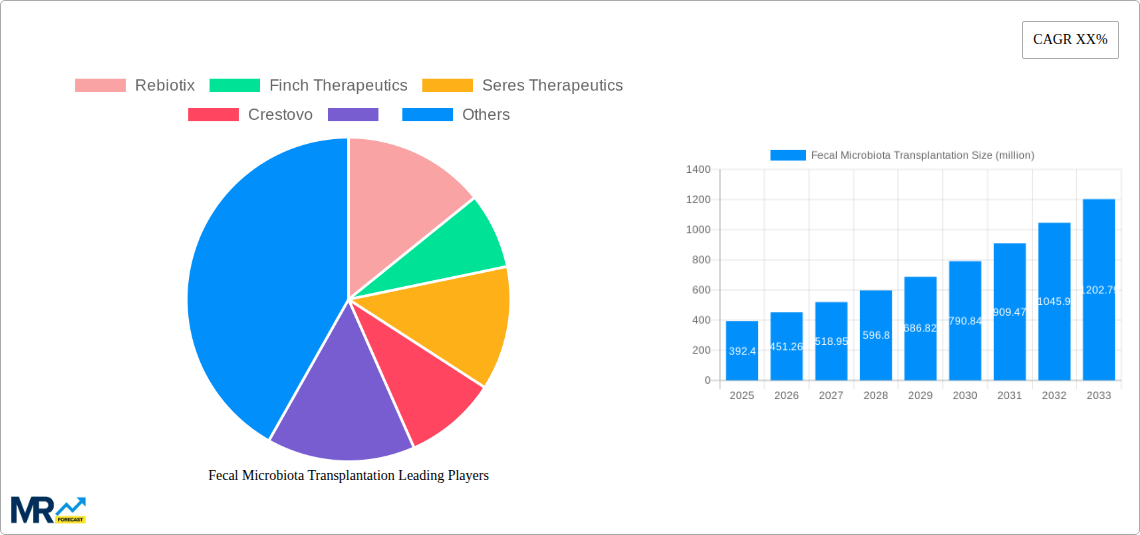
The Fecal Microbiota Transplantation (FMT) market is experiencing a transformative period, poised for significant expansion driven by a deeper understanding of the human microbiome's intricate role in health and disease. Over the Study Period of 2019-2033, the market has witnessed a steady upward trajectory, with the Base Year of 2025 serving as a crucial point for estimating future growth. The Estimated Year of 2025, aligning with the Base Year, indicates an optimistic outlook. The Forecast Period, spanning 2025-2033, anticipates a compound annual growth rate (CAGR) that will propel the market value from millions into the billions. The Historical Period of 2019-2024 laid the foundational groundwork, characterized by increasing clinical validation, regulatory progress, and a growing awareness of FMT's therapeutic potential. Initial market valuations in the early Historical Period were modest, likely in the tens of millions, but have seen substantial growth. By the end of the Historical Period, the market value had climbed into the hundreds of millions, fueled by promising Phase 2 and Phase 3 trial results for various indications. Looking ahead to the Forecast Period, the market is projected to reach values in the high hundreds of millions, potentially surpassing a billion dollars by the end of 2033. This growth is not just about increasing patient numbers but also about the expansion of applications beyond the well-established Clostridium Difficile Infections (CDI). The emergence of well-characterized FMT products and standardized protocols is a key driver, increasing patient and physician confidence. Furthermore, advancements in manufacturing and delivery methods are contributing to market scalability and accessibility. The market's evolution reflects a paradigm shift in medicine, moving towards personalized and microbiome-targeted therapies, with FMT at the forefront of this revolution. The initial market penetration was limited, but as clinical evidence mounts and regulatory pathways become clearer, the demand for FMT solutions is expected to surge exponentially. The value proposition of FMT, particularly its potential to offer a durable and less invasive alternative to traditional treatments for recurrent CDI, is a significant factor in its market growth. Beyond CDI, the exploration of FMT for a myriad of other conditions, from metabolic disorders to neurological diseases, is opening up vast new avenues for market expansion. Early-stage research and development efforts, even if not immediately translating into market revenue, contribute significantly to the overall market dynamism by signaling future growth potential. The increasing investment in research and development by key players, coupled with the establishment of dedicated FMT centers, further solidifies the market's upward trajectory. The evolving regulatory landscape, though sometimes a challenge, is also a crucial trend as it aims to ensure product safety and efficacy, thereby fostering broader clinical adoption and market trust. The market's trajectory demonstrates a clear transition from an experimental therapy to a recognized and increasingly adopted treatment modality.
Several key forces are driving the remarkable growth and increasing adoption of Fecal Microbiota Transplantation (FMT). Foremost among these is the compelling clinical evidence demonstrating its efficacy, particularly in treating recurrent Clostridium Difficile Infections (CDI). The high cure rates achieved by FMT, often exceeding 90% in challenging CDI cases, present a significant advantage over conventional antibiotic therapies that frequently lead to relapse. This success has not only established FMT as a gold standard for refractory CDI but has also ignited considerable interest in its application for a wider spectrum of gastrointestinal disorders. Beyond CDI, a growing body of research is exploring FMT's potential in conditions like Inflammatory Bowel Disease (IBD), Irritable Bowel Syndrome (IBS), and other gut-related ailments. The hypothesis is that by restoring a healthy and diverse gut microbiome, FMT can modulate immune responses, reduce inflammation, and improve gut barrier function, offering a novel therapeutic approach for these complex diseases. Furthermore, the burgeoning scientific understanding of the gut-brain axis is opening up exciting possibilities for FMT in neurological and psychiatric disorders. Studies investigating FMT for Parkinson Disease, Autism, and even certain mental health conditions are gaining traction, driven by observations that microbiome dysbiosis is frequently associated with these conditions. The development of standardized, well-characterized FMT products, moving away from traditional donor screening and preparation methods, is another significant propellant. Companies are investing heavily in developing consistent, safe, and reproducible FMT formulations, which will be crucial for regulatory approval and widespread clinical use. The increasing investment from venture capital and pharmaceutical giants in FMT-focused companies and research initiatives further underscores the market's potential and the confidence of industry stakeholders. This financial influx is accelerating clinical trials, fostering innovation, and ultimately bringing FMT closer to mainstream medical practice. As regulatory bodies worldwide refine pathways for FMT approval, it signals a more streamlined route for bringing these therapies to patients, thereby removing significant barriers to market entry and expansion. The increasing public awareness and patient advocacy for microbiome-based therapies also contribute to the market's momentum, creating demand and encouraging healthcare providers to explore FMT as a viable treatment option.
Despite its promising trajectory, the Fecal Microbiota Transplantation (FMT) market faces several significant hurdles that could temper its growth. A primary challenge revolves around the regulatory landscape. While progress is being made, clear and consistent regulatory pathways for FMT products and their approval remain under development in many regions. This ambiguity can lead to delays in clinical trials, hinder market entry, and create uncertainty for both developers and healthcare providers. Ensuring the safety and standardization of FMT is paramount. Traditional FMT relies on donor screening and fecal material processing, which can introduce variability and potential risks, including the transmission of infectious agents. The development of off-the-shelf, bankable FMT products with rigorous quality control and standardization is crucial but presents complex manufacturing and logistical challenges. This includes establishing robust screening protocols for donors, ensuring the consistent composition of microbial consortia, and developing stable formulations for storage and administration. The perception and acceptance of FMT among healthcare professionals and the general public also represent a considerable restraint. The very nature of FMT, involving fecal material, can evoke strong psychological barriers and concerns about hygiene and safety, despite its demonstrated therapeutic benefits. Educating physicians and patients about the scientific rationale, efficacy, and safety of FMT is an ongoing and vital process. Furthermore, the reimbursement landscape for FMT can be complex and inconsistent. While FMT for recurrent CDI is increasingly covered by insurance, its application for other indications may not be as readily reimbursed, limiting patient access and market penetration. The cost of developing and manufacturing standardized FMT products, along with the specialized infrastructure and trained personnel required for administration, can also contribute to higher treatment costs, posing a barrier for widespread adoption, particularly in resource-limited settings. The lack of long-term data and extensive clinical trials for many emerging FMT applications, beyond CDI, also presents a restraint. While initial results are promising, robust, large-scale, multi-center studies are needed to definitively establish efficacy and safety for conditions like Parkinson Disease, Obesity, Diabetes Mellitus, and Autism. Finally, the potential for unforeseen adverse events, although rare, necessitates continued vigilance and comprehensive post-market surveillance.
The Fecal Microbiota Transplantation (FMT) market is characterized by distinct regional dynamics and segment dominance, with North America and Europe currently leading the charge.
North America:
Europe:
Emerging Markets (Asia-Pacific, Rest of the World):
The dominance of Clostridium Difficile Infections (CDI) as an application segment is undeniable, being the most established and widely recognized indication for FMT. However, the future growth is anticipated to be driven by the exploration of FMT in Parkinson Disease, Obesity, and Diabetes Mellitus, as well as a broader range of "Other" conditions. In terms of developmental phases, Phase-3 and Phase-4 studies represent the current market leaders in terms of investment and activity in established regions, while Phase-1 and Phase-2 are crucial for exploring new frontiers and demonstrating initial efficacy in emerging markets and novel applications.
Several factors are acting as potent growth catalysts for the Fecal Microbiota Transplantation (FMT) industry. The escalating incidence of antibiotic-resistant infections, particularly recurrent Clostridium Difficile Infections, directly fuels the demand for highly effective alternative therapies like FMT. Furthermore, the rapid advancements in scientific understanding of the gut microbiome's profound influence on systemic health, including immune function, metabolism, and neurological processes, are opening up vast new therapeutic avenues for FMT. The development of standardized, commercially available FMT products by key players like Rebiotix, Finch Therapeutics, Seres Therapeutics, and Crestovo is a critical catalyst, enhancing safety, reproducibility, and accessibility. Increased investment from venture capital and pharmaceutical companies signifies growing confidence in FMT's market potential, accelerating research and development. Finally, evolving regulatory pathways by agencies like the FDA are streamlining approvals, paving the way for broader clinical adoption.
This report offers an in-depth exploration of the Fecal Microbiota Transplantation (FMT) market, providing a holistic view of its current landscape and future potential. It meticulously analyzes market trends, identifying key drivers and restraints that shape its trajectory. The report delves into the dominant regions and segments, offering granular insights into their market share and growth prospects. Furthermore, it highlights the significant growth catalysts propelling the industry forward and profiles the leading players actively shaping the FMT ecosystem. With a comprehensive study period from 2019-2033, including a base year of 2025 and a detailed forecast period from 2025-2033, this report provides actionable intelligence for stakeholders seeking to navigate this dynamic and rapidly evolving therapeutic area. The analysis encompasses various phases of clinical development (Phase-I to Phase-IV) and a broad spectrum of applications, from established treatments for Clostridium Difficile Infections to emerging potential in Parkinson Disease, Obesity, Diabetes Mellitus, Autism, and beyond.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Rebiotix, Finch Therapeutics, Seres Therapeutics, Crestovo, .
The market segments include Type, Application.
The market size is estimated to be USD 392.4 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Fecal Microbiota Transplantation," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Fecal Microbiota Transplantation, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.