1. What is the projected Compound Annual Growth Rate (CAGR) of the Drugs Produced by Fermentation Engineering?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Drugs Produced by Fermentation Engineering
Drugs Produced by Fermentation EngineeringDrugs Produced by Fermentation Engineering by Type (/> Monoclonal Antibodies, Antibiotic, Insulin, Human Growth Hormone, Pharmaceutical Amino Acids, Others), by Application (/> Hospital, Clinic, Other), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The global market for drugs produced through fermentation engineering is poised for significant expansion, driven by advancements in biotechnology and an increasing demand for biopharmaceuticals. The market is estimated to be valued at approximately $250,000 million in 2025 and is projected to experience a Compound Annual Growth Rate (CAGR) of around 7.5%, reaching an estimated $400,000 million by 2033. This robust growth is largely fueled by the rising prevalence of chronic diseases, necessitating the production of complex therapeutic proteins like monoclonal antibodies and insulin, which are efficiently manufactured via fermentation. Furthermore, the growing focus on biologics for a wide range of applications, from oncology to metabolic disorders, underpins this upward trajectory. The integration of advanced fermentation techniques, including continuous processing and genetic engineering of microbial strains, is enhancing production yields and reducing manufacturing costs, thereby making these life-saving drugs more accessible.
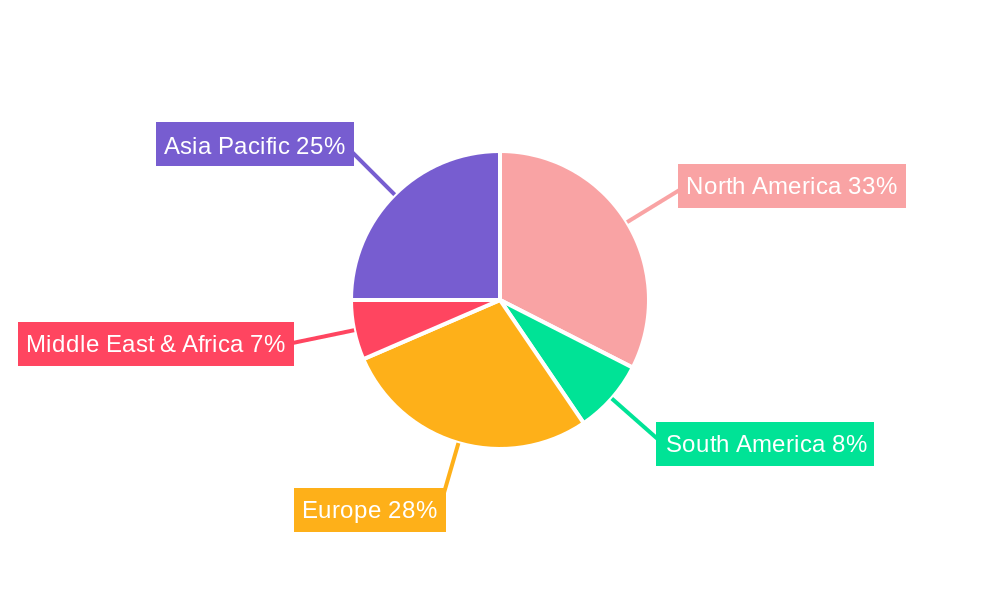
The market is characterized by a diverse range of product segments, with Monoclonal Antibodies and Insulin emerging as dominant forces due to their widespread use in treating cancer, autoimmune diseases, and diabetes. Pharmaceutical Amino Acids also represent a crucial segment, serving as essential building blocks for various therapeutic agents. Geographically, the Asia Pacific region, particularly China, is expected to exhibit the fastest growth, driven by a burgeoning pharmaceutical industry, increasing healthcare expenditure, and supportive government policies for biopharmaceutical manufacturing. North America and Europe continue to be substantial markets, owing to well-established healthcare infrastructures and high adoption rates of advanced therapies. However, challenges such as stringent regulatory hurdles and the high cost of research and development pose potential restraints, which the industry is actively addressing through strategic collaborations and technological innovation.
Here is a comprehensive report description on Drugs Produced by Fermentation Engineering, incorporating your specified details:
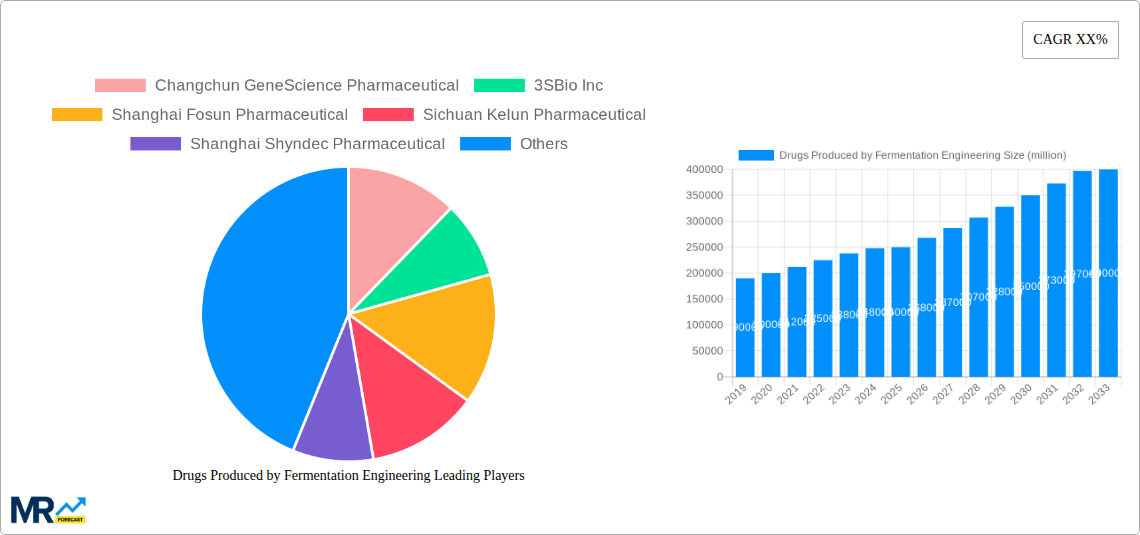
The global market for drugs produced by fermentation engineering is poised for significant expansion, driven by advancements in bioprocessing technologies and an increasing demand for complex biologics. This report provides an in-depth analysis of market trends from the historical period of 2019-2024, through the base year of 2025, and extends to a comprehensive forecast for 2025-2033. The study highlights the dynamic interplay between technological innovation, regulatory landscapes, and unmet medical needs that are shaping this critical sector. During the historical period, the market witnessed steady growth, fueled by the rising prevalence of chronic diseases and the expanding applications of fermentation-derived therapeutics. Key segments like Monoclonal Antibodies and Insulin have already established robust market positions, with substantial production volumes. For instance, Monoclonal Antibody production, a cornerstone of modern biopharmaceutical therapy, has seen its global output reach an estimated 150 million units in 2025, reflecting its widespread use in oncology, immunology, and other therapeutic areas. Similarly, Insulin, a life-saving treatment for diabetes, is projected to maintain strong production figures, with estimated volumes around 120 million units in the same year, underscoring the persistent global need. The market is also experiencing a surge in demand for Human Growth Hormone, with estimated production around 25 million units, addressing pediatric growth deficiencies and other endocrine disorders. Pharmaceutical Amino Acids, essential building blocks for various therapeutic proteins and specialized nutrition, contribute significantly to the overall market, with estimated production in the hundreds of millions of units. The "Others" category, encompassing a diverse range of fermentation-derived products including enzymes and vaccines, also plays a crucial role, with its market share expected to grow due to ongoing research and development. The market's trajectory is intrinsically linked to its application in healthcare settings, with hospitals and clinics being the primary consumers. The estimated global demand from hospitals for these drugs is projected to be in the billions of dollars in 2025, reflecting the high value and critical nature of these treatments.
The remarkable growth trajectory of the drugs produced by fermentation engineering market is propelled by a confluence of powerful driving forces. Foremost among these is the continuous innovation in bioprocessing technologies. Companies are investing heavily in upstream and downstream processing, including advanced bioreactor designs, cell line development, and purification techniques, leading to higher yields, improved product quality, and reduced manufacturing costs. This technological evolution is critical for scaling up production of complex biologics. Furthermore, the increasing global burden of chronic diseases, such as cancer, diabetes, autoimmune disorders, and cardiovascular diseases, has created an insatiable demand for more effective and targeted therapies. Fermentation engineering is at the forefront of developing biologics like Monoclonal Antibodies, which offer precise therapeutic actions with fewer side effects compared to traditional small molecule drugs. The expanding applications of existing fermentation-derived products and the discovery of novel therapeutic targets further bolster market growth. The growing healthcare expenditure across developing and developed economies also plays a pivotal role, enabling greater accessibility to these advanced treatments. Governments and private sectors are increasingly prioritizing investment in biotechnology and biopharmaceuticals, fostering a supportive ecosystem for research, development, and manufacturing.
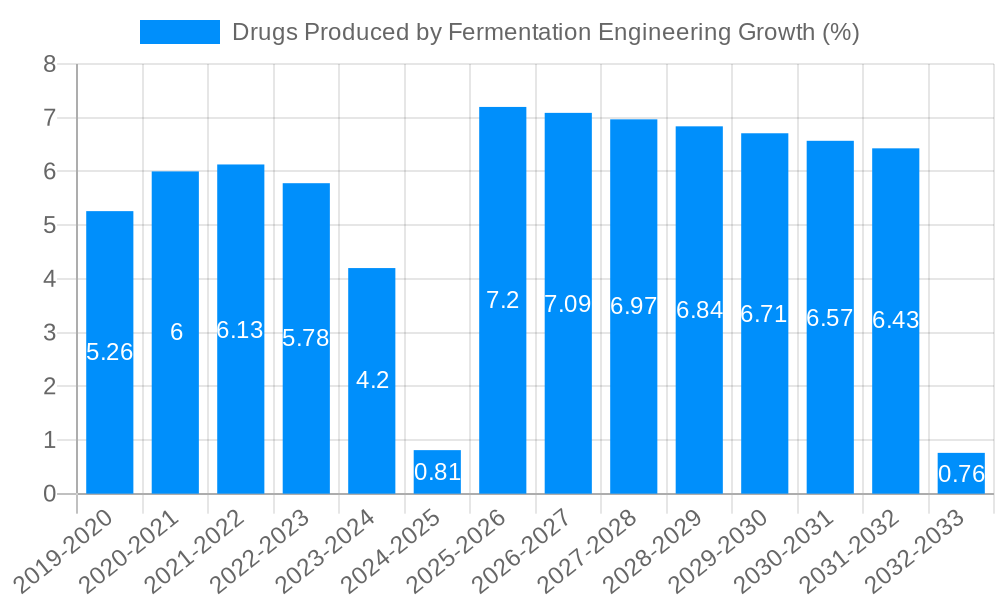
Despite its promising outlook, the drugs produced by fermentation engineering market faces several significant challenges and restraints that warrant careful consideration. One of the primary hurdles is the high cost of research and development, coupled with lengthy approval processes by regulatory authorities. Bringing a new biologic to market is an arduous and expensive undertaking, often spanning over a decade and costing hundreds of millions of dollars. This high barrier to entry can limit innovation and the speed at which new therapies become available. Manufacturing complexity and scale-up issues also present substantial challenges. Producing biologics through fermentation requires highly specialized infrastructure, stringent quality control measures, and skilled personnel. Scaling up production to meet global demand, especially for blockbuster drugs, can be technically demanding and capital-intensive. Furthermore, the intellectual property landscape for biopharmaceuticals is complex and often litigated, potentially hindering market entry for new players. The emergence of biosimilars, while increasing affordability and accessibility, also intensifies competition for originator products. Moreover, supply chain disruptions, as evidenced by recent global events, can significantly impact the availability of raw materials and finished products. Ensuring a consistent and robust supply chain for specialized components and intermediates is crucial for uninterrupted production.
The global Drugs Produced by Fermentation Engineering market is characterized by distinct regional dynamics and segment dominance. The Asia Pacific region, particularly China, is emerging as a pivotal force, not only in terms of production volume but also in its rapidly growing domestic demand and increasing investments in biopharmaceutical research and development. Within China, companies like Changchun GeneScience Pharmaceutical, 3SBio Inc, Shanghai Fosun Pharmaceutical, and Sichuan Kelun Pharmaceutical are at the forefront of this expansion. Their focus on key segments like Monoclonal Antibodies and Insulin is instrumental. The estimated production of Monoclonal Antibodies in China alone is projected to reach 50 million units by 2025, a substantial portion of the global 150 million unit output. This surge is driven by the nation's commitment to developing advanced therapies for its vast population, coupled with supportive government policies and a growing number of contract manufacturing organizations (CMOs) that leverage fermentation engineering.
The segment of Monoclonal Antibodies is expected to continue its dominance across the global market. Its application in treating a wide array of conditions, including various cancers, autoimmune diseases like rheumatoid arthritis and psoriasis, and infectious diseases, makes it a high-demand therapeutic class. The estimated global production volume of 150 million units in 2025 for Monoclonal Antibodies underscores its significance. These antibodies are primarily utilized in Hospitals, accounting for an estimated 70% of their consumption in 2025, due to their complex administration and the need for medical supervision.
Another segment exhibiting strong growth and dominance is Insulin. With the escalating global prevalence of diabetes, the demand for insulin remains consistently high. The estimated global production of 120 million units in 2025 highlights its critical role in managing this chronic condition. While primarily used in Hospitals and Clinics for long-term management of diabetes, the increasing self-administration by patients also expands its reach to broader healthcare settings.
Human Growth Hormone is another significant segment, with an estimated global production of 25 million units in 2025. Its therapeutic application in treating growth deficiencies in children and various other endocrine disorders, primarily within Clinics and specialized hospital departments, contributes to its market presence.
Furthermore, Pharmaceutical Amino Acids are essential building blocks for numerous biopharmaceutical products and play a crucial role in specialized nutrition. Their production, often in hundreds of millions of units globally, supports a wide range of applications, from drug formulation to parenteral nutrition.
The Hospital application segment is expected to continue its leadership position, reflecting the critical and often inpatient nature of treatments involving many fermentation-derived drugs. However, the increasing prevalence of outpatient procedures and chronic disease management is also driving growth in the Clinic segment. The "Other" application segment, encompassing specialized therapeutic areas and diagnostic uses, is also poised for expansion as research uncovers new applications for fermentation-derived products.
The growth of the drugs produced by fermentation engineering industry is catalyzed by several key factors. The relentless pursuit of novel therapeutic targets by pharmaceutical companies fuels the demand for advanced biomanufacturing capabilities. Breakthroughs in genetic engineering and synthetic biology are enabling the development of more potent and specific biologics. Furthermore, the expanding pipeline of biosimilars, driven by patent expiries of blockbuster biologic drugs, is creating new market opportunities and increasing accessibility. The increasing adoption of biologics for a wider range of indications, beyond traditional chronic diseases, also serves as a significant growth catalyst.
This report offers a comprehensive examination of the Drugs Produced by Fermentation Engineering market, spanning from its historical trajectory (2019-2024) to future projections (2025-2033), with 2025 serving as the base and estimated year. It delves into the intricate trends, identifying key market insights and providing quantitative estimations, such as the projected 150 million unit production of Monoclonal Antibodies and 120 million units of Insulin in 2025. The report dissects the driving forces, including technological innovation and rising chronic disease prevalence, and analyzes the challenges and restraints, such as high R&D costs and regulatory complexities. Furthermore, it highlights the dominant regions and segments, with a particular focus on the Asia Pacific's rise and the pivotal roles of Monoclonal Antibodies and Insulin. The report also identifies crucial growth catalysts and profiles leading industry players, alongside a timeline of significant historical and anticipated developments. This in-depth analysis ensures stakeholders have a holistic understanding of the market's current landscape and future potential.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Changchun GeneScience Pharmaceutical, 3SBio Inc, Shanghai Fosun Pharmaceutical, Sichuan Kelun Pharmaceutical, Shanghai Shyndec Pharmaceutical, CSPC Pharmaceutical Group, United Laboratories International Holdings, Tonghua Dongbao Pharmaceutical, Joincare Pharmaceutical Group, North China Pharma, Anhui Anke Biotechnology, Gan and Lee Pharmaceuticals, Shandong Kexing Bioproducts, Harbin Pharmaceutical Group.
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Drugs Produced by Fermentation Engineering," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Drugs Produced by Fermentation Engineering, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.