1. What is the projected Compound Annual Growth Rate (CAGR) of the COVID-19 Rapid Antigen Test Kits?
The projected CAGR is approximately 6.9%.
 COVID-19 Rapid Antigen Test Kits
COVID-19 Rapid Antigen Test KitsCOVID-19 Rapid Antigen Test Kits by Application (Hospital, Home, School, Third-Party Detection Institution, Others), by Type (Fluorescence Immunochromatography, Colloidal Gold Immunochromatography, Latex Immunochromatography, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
The COVID-19 pandemic spurred significant growth in the rapid antigen test kit market. The market, valued at approximately $28.84 billion in 2025, is projected to experience substantial growth over the forecast period (2025-2033). While a precise CAGR is unavailable, considering the initial surge in demand followed by a gradual stabilization as vaccination rates increased and the pandemic transitioned to an endemic phase, a conservative estimate of a 5-7% CAGR is reasonable. This moderate growth reflects continued demand for rapid testing in healthcare settings, as well as for at-home testing, driven by the ongoing need for early detection and infection control. Key drivers include the ease of use and rapid turnaround time of these tests, making them suitable for mass testing initiatives and personal use. However, factors like evolving virus variants, the increasing availability of more sensitive PCR tests, and the potential for false negative results pose certain restraints to market expansion. The market segmentation, while not explicitly defined, likely involves variations based on test format (e.g., nasal swab, saliva), intended user (professional healthcare setting, at-home use), and geographical region. The significant number of companies involved (Abbott, Quidel, ACCESS BIO, and many others) highlights the competitive landscape, with manufacturers focusing on innovation, product differentiation (such as improved sensitivity and specificity), and expanding distribution networks to maintain a strong market position.
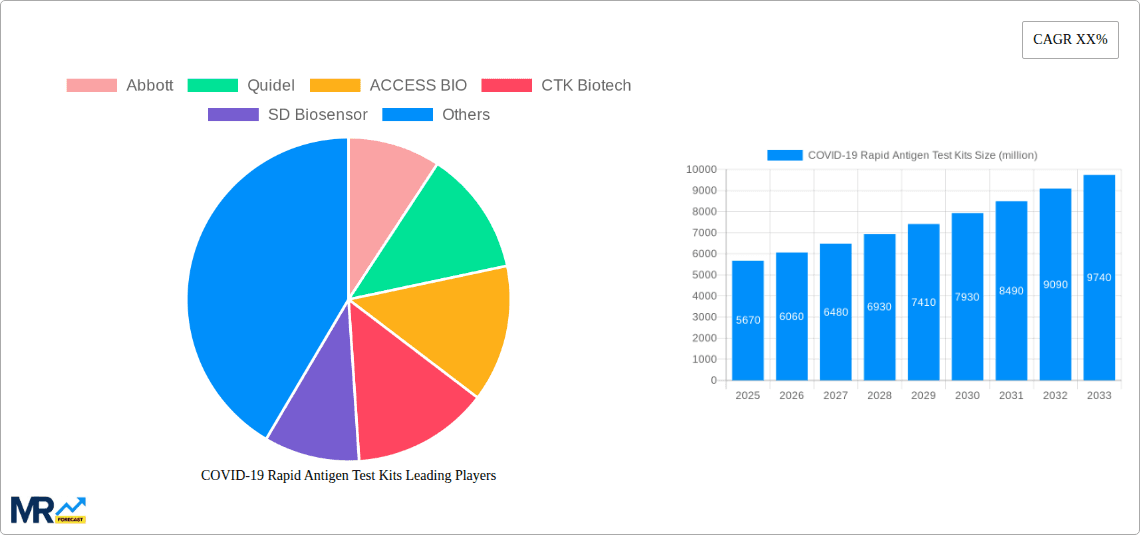

The competitive landscape is intense, with numerous established players and emerging companies vying for market share. Established players like Abbott and Roche leverage their existing infrastructure and brand recognition, while newer entrants focus on technological advancements and cost-effective solutions. Geographical variations in market penetration are expected, with developed nations exhibiting higher per capita usage compared to developing countries. Future growth will hinge on factors such as the emergence of new variants, continued public health initiatives, and ongoing advancements in rapid antigen test technology. The market is expected to transition from the pandemic-driven surge towards a more stable growth trajectory, driven by a combination of routine testing, surveillance needs, and potential for integration into broader healthcare systems.
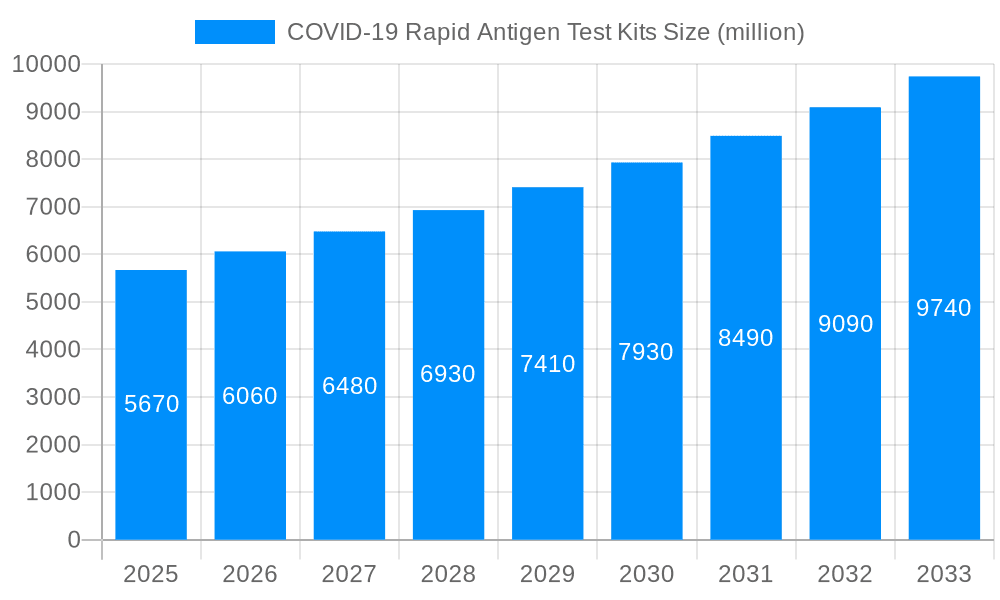

The COVID-19 pandemic spurred unprecedented demand for rapid antigen test kits, propelling the market to a multi-billion-dollar industry. From 2019 to 2024 (the historical period), the market witnessed explosive growth, with sales exceeding tens of millions of units annually. The estimated market size in 2025 surpasses hundreds of millions of units, indicating sustained, albeit decelerating, demand. The forecast period (2025-2033) projects continued market expansion, though at a more moderate pace compared to the initial surge, driven by ongoing needs for surveillance, outbreaks, and potential future pandemics. This transition reflects a shift from emergency response to more integrated disease management strategies. Key market insights reveal a growing preference for user-friendly, at-home tests, pushing manufacturers to innovate in terms of accuracy, speed, and accessibility. The market is also becoming increasingly segmented, with variations in test formats, target populations (e.g., children, healthcare workers), and pricing points. The competition among numerous manufacturers, both established and emerging, is intense, leading to continuous improvements in product quality and cost-effectiveness. The market's trajectory showcases a transition from pandemic-driven urgency to a more sustainable model focused on public health preparedness and ongoing disease monitoring. The availability of millions of units annually demonstrates the global scale of this market. The increasing integration of these tests into routine healthcare practices, coupled with advancements in testing technology, is key to understanding the long-term trends shaping the market.
Several factors fuel the growth of the COVID-19 rapid antigen test kits market. The primary driver remains the ongoing need for rapid and convenient diagnostic tools to manage COVID-19 and similar respiratory illnesses. The speed and ease of use of these tests are significantly advantageous compared to traditional PCR tests, enabling quicker identification of infected individuals and facilitating prompt isolation and treatment. Furthermore, the relatively low cost of rapid antigen tests, especially when purchased in bulk, makes them accessible to a wider population and various healthcare settings, including home testing. Government initiatives and public health programs promoting widespread testing have also contributed significantly to market growth. The development of increasingly accurate and sensitive rapid antigen tests further enhances their appeal to both healthcare professionals and the general public. Moreover, the potential for repurposing these tests for the detection of other infectious diseases offers a promising avenue for future growth, particularly given the lessons learned during the COVID-19 pandemic regarding the importance of rapid diagnostics. Finally, continuous technological advancements are driving the production of more efficient, reliable, and cost-effective tests, sustaining the market's momentum.
Despite the substantial growth, the COVID-19 rapid antigen test kit market faces several challenges. The accuracy of rapid antigen tests, while improving, is generally lower compared to PCR tests, leading to potential false-negative results. This necessitates careful interpretation of test results and potentially requires confirmatory testing in certain scenarios. The fluctuating demand, initially driven by pandemic-level surges, is now transitioning to a more stable, though still significant, level. This fluctuating demand makes production planning and supply chain management complex for manufacturers. Regulatory hurdles and the need for continuous product approvals in different countries also pose significant barriers to market entry and expansion for manufacturers. The emergence of new viral variants and their potential impact on test sensitivity add another layer of complexity and necessitates ongoing efforts in test development and validation. Furthermore, cost considerations, especially for low-income populations and resource-limited settings, can hinder widespread adoption. Addressing these challenges through continuous innovation, improved regulatory processes, and investment in public health infrastructure is crucial for the sustainable growth of the market.
The market's dominance is spread across several regions and segments:
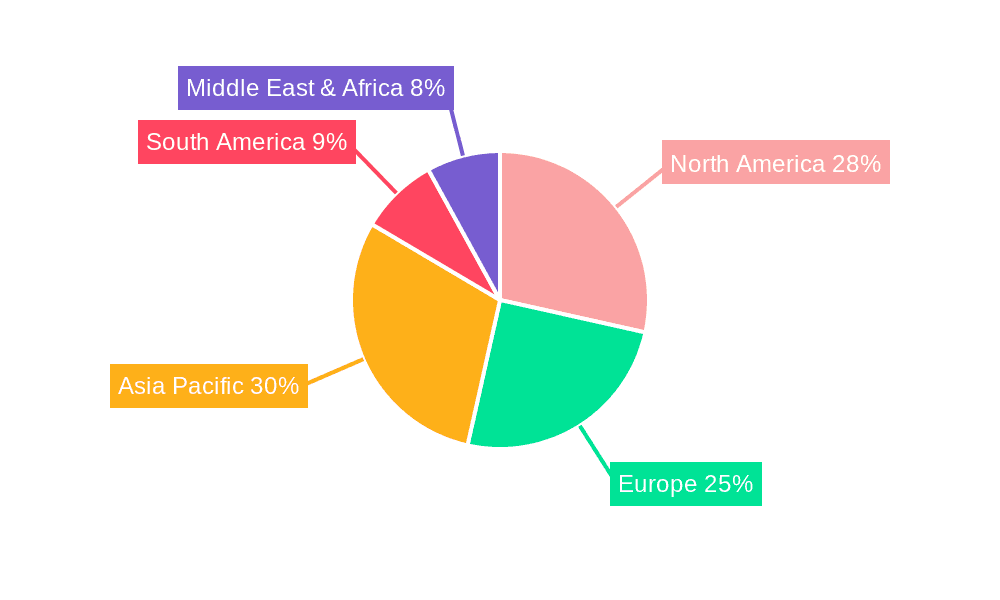
North America and Europe: These regions, characterized by well-established healthcare infrastructure and high per capita income, were early adopters and remain significant consumers of rapid antigen test kits. The high demand fueled by robust healthcare systems and higher awareness led to rapid market expansion and adoption of advanced technologies. Millions of units were distributed and used in these regions during the peak pandemic period.
Asia-Pacific: The Asia-Pacific region exhibits a diverse market landscape, driven by population density and variable economic conditions. While initial adoption was slower compared to North America and Europe, the market has experienced substantial growth due to rising awareness, expanding healthcare infrastructure, and increasing affordability. Significant sales figures in millions of units reflect this rapid expansion.
At-Home Testing Segment: The segment of at-home, over-the-counter tests has experienced phenomenal growth, driven by convenience and ease of access. The ability to self-administer tests contributed to widespread testing and the quick identification of infected individuals, contributing substantially to sales figures in the millions.
Professional Use Segment: Hospitals, clinics, and other healthcare facilities continue to utilize rapid antigen tests for rapid screening and diagnosis, ensuring this segment remains a substantial contributor to overall market revenue. While the volume might be smaller than at-home testing in terms of units sold, the pricing structure contributes significantly to market value.
The dominance shifts across regions and segments depending on the stage of the pandemic, economic conditions, and the relative adoption of at-home versus professional testing.
The COVID-19 rapid antigen test kit industry's growth is catalyzed by several converging factors. Continued refinement of test accuracy and sensitivity, coupled with the emergence of tests capable of detecting multiple respiratory viruses, expands their utility beyond COVID-19. Government funding for research and development and incentives for widespread testing remain essential in promoting market expansion. Moreover, the ongoing development of cost-effective, point-of-care testing technologies drives broader accessibility and adoption, boosting sales and solidifying the market position of this technology for long-term public health management.
This report provides a comprehensive overview of the COVID-19 rapid antigen test kits market, spanning the period from 2019 to 2033. It offers in-depth analysis of market trends, growth drivers, challenges, and key players, with a focus on sales figures in the millions of units annually. The report segments the market by region, segment, and key players, providing valuable insights for industry stakeholders seeking to understand the market dynamics and opportunities within this rapidly evolving sector. The comprehensive analysis offers forecasts for future growth and provides a clear understanding of the factors influencing the market’s future trajectory.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6.9% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 6.9%.
Key companies in the market include Abbott, Quidel, ACCESS BIO, CTK Biotech, SD Biosensor, Ellume, InBios, Celltrion, AESKU.GROUP, ROCHE, BD, OraSure Technologies, Clip Health, LumiraDx, Princeton BioMeditech Corporation, RapiGEN Inc., Orient Gene, Wondfo Biotech, Ihealth (Andon Health), Wantai BioPharm, Biotest Biotech, Shenzhen YHLO Biotech, Vazyme Biotech, EasyDiagnosis Biomedicine, Beijing Hotgen Biotech, Assure Tech, Hangzhou AllTest Biotech, ACON Biotech, BGI Genomics, Lepu Medical Technology, Jinwofu Bioengineering Technology, Phase Scientific, .
The market segments include Application, Type.
The market size is estimated to be USD XXX N/A as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in N/A and volume, measured in K.
Yes, the market keyword associated with the report is "COVID-19 Rapid Antigen Test Kits," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the COVID-19 Rapid Antigen Test Kits, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.