1. What is the projected Compound Annual Growth Rate (CAGR) of the Covid-19 Antigen Test Kits?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Covid-19 Antigen Test Kits
Covid-19 Antigen Test KitsCovid-19 Antigen Test Kits by Type (Very High Sensitivity(Clinical Sensitivity Greater than 80% PPA), High Sensitivity(Clinical Sensitivity Greater than 90% PPA), Acceptable Sensitivity(Clinical Sensitivity Greater than 95% PPA)), by Application (Government Purchases, Individual Purchases), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The global Covid-19 Antigen Test Kits market is projected to experience robust growth, reaching an estimated $5,800 million by 2025, with a Compound Annual Growth Rate (CAGR) of approximately 12% projected through 2033. This sustained expansion is driven by several critical factors, including the ongoing need for rapid and accessible diagnostic solutions for SARS-CoV-2, governmental procurement programs aimed at public health surveillance and outbreak management, and an increasing demand from individual consumers for at-home testing. The prevalence of highly sensitive test types, particularly those exceeding 90% and 95% PPA (Positive Percent Agreement), is a significant trend, as these offer greater confidence in diagnostic accuracy. Furthermore, the decentralization of testing beyond clinical settings into community centers and homes continues to fuel market penetration.
Despite the diminishing acute phase of the pandemic, the market for Covid-19 Antigen Test Kits remains dynamic due to the emergence of new variants, the persistent threat of future outbreaks, and the integration of antigen testing into broader respiratory illness diagnostic strategies. Restraints, such as evolving regulatory landscapes, potential pricing pressures due to market saturation, and the development of more advanced or alternative diagnostic technologies, are anticipated to moderate growth in specific segments. However, the established infrastructure for antigen testing, coupled with continued innovation in sensitivity and ease of use, will likely ensure its continued relevance. Key companies like Abbott, SD Biosensor, and Access Bio are at the forefront, leveraging their established supply chains and R&D capabilities to capitalize on these evolving market dynamics. The Asia Pacific region, particularly China and India, is expected to be a significant contributor to market volume due to high population density and robust manufacturing capabilities.
Here is a unique report description on Covid-19 Antigen Test Kits, incorporating your specified elements:
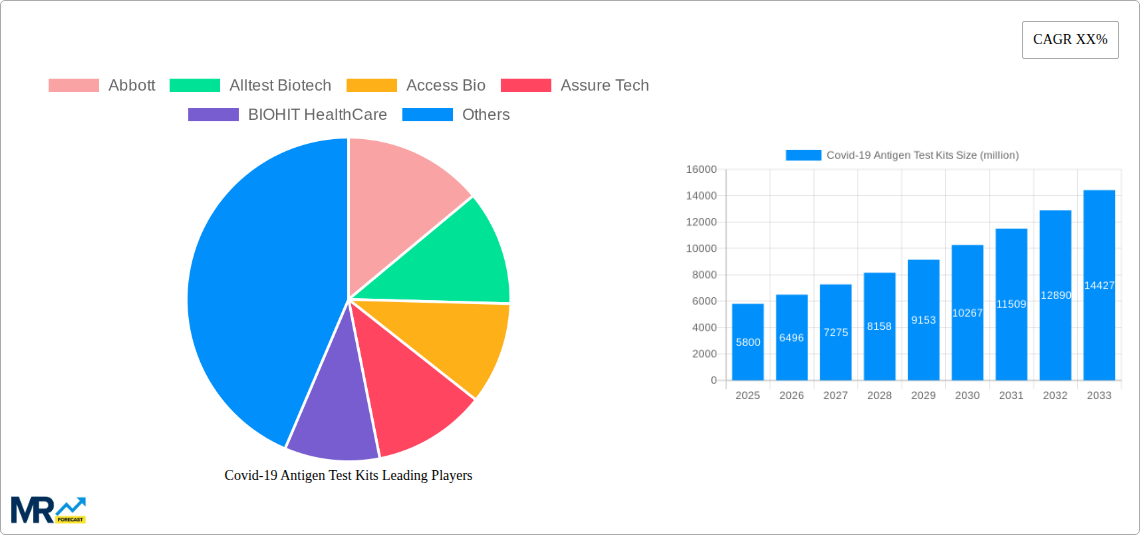
XXX The Covid-19 Antigen Test Kit market, a critical tool in the global pandemic response, has undergone a dramatic transformation since its inception in 2019. The historical period (2019-2024) witnessed an unprecedented surge in demand, driven by the immediate need for rapid and accessible diagnostics. This period saw the market evolve from nascent stages to a mature, albeit volatile, landscape. By the base year of 2025, the market has established a significant footprint, with an estimated 3,500 million units in sales. The forecast period (2025-2033) is projected to see continued, albeit more measured, growth. This expansion will be fueled by the ongoing need for surveillance, early detection in symptomatic individuals, and integration into routine public health strategies. The shift from emergency response to endemic management will redefine the market's dynamics. Manufacturers have invested heavily in research and development, leading to a diversification of product offerings and improvements in sensitivity and specificity. The estimated sales for the year 2025 are expected to reach 3,800 million units, indicating a steady upward trajectory. Innovation in testing platforms, including at-home testing solutions and point-of-care devices, will be a defining characteristic of this market. Furthermore, the development of multiplex tests capable of detecting multiple respiratory pathogens simultaneously, including SARS-CoV-2, will also contribute to market expansion. Regulatory approvals and evolving testing guidelines across different regions will play a pivotal role in shaping the demand for various types of antigen test kits. The competitive landscape is robust, with established players and emerging companies vying for market share, all contributing to the overall market size and growth potential.
The sustained demand for Covid-19 Antigen Test Kits is propelled by a confluence of factors that underscore their indispensable role in public health infrastructure. Foremost among these is the ongoing global necessity for rapid and accessible diagnostic solutions. The ability of antigen tests to provide quick results at the point of care or even in the home environment has been instrumental in facilitating early detection, isolation, and containment of the virus. This immediacy significantly aids in curbing transmission chains, a critical objective even as the pandemic shifts into an endemic phase. Furthermore, government initiatives and public health policies worldwide continue to support widespread testing programs. These programs are crucial for surveillance, monitoring outbreak hotspots, and ensuring the safety of public spaces, workplaces, and educational institutions. The increasing recognition of antigen tests as a viable complement or, in certain scenarios, alternative to PCR testing, due to their cost-effectiveness and speed, further bolsters their adoption. The convenience and affordability of antigen test kits empower individuals to take proactive measures for their health and that of their communities. This decentralized approach to testing, moving beyond centralized laboratories, has been a key driver of market growth and accessibility.
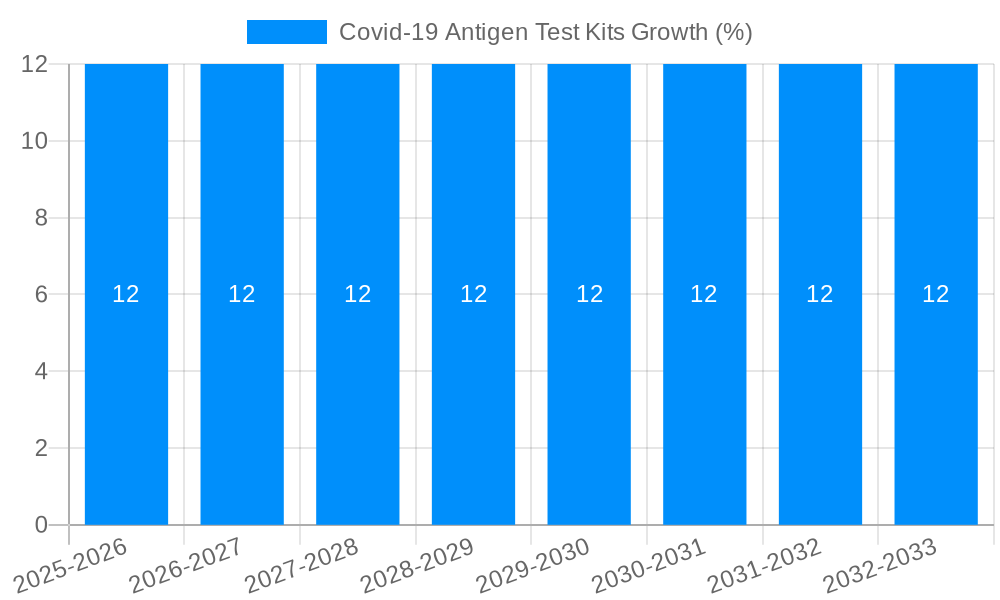
Despite the strong market drivers, the Covid-19 Antigen Test Kits sector faces several significant challenges and restraints that could temper its growth trajectory. A primary concern revolves around the inherent limitations in sensitivity and specificity compared to molecular (PCR) tests. While antigen tests have improved, a proportion of false negatives or false positives can still occur, leading to potential under- or over-diagnosis and consequently, suboptimal public health responses. This can erode public trust and lead to a preference for more accurate, albeit slower and more expensive, molecular tests in certain critical settings. Furthermore, the market is highly price-sensitive, with intense competition among manufacturers driving down profit margins. This can make it challenging for smaller players or those investing heavily in R&D to sustain their operations. The evolving nature of the virus, including the emergence of new variants, also poses a challenge. Antigen tests need to maintain efficacy against circulating strains, requiring continuous adaptation and revalidation, which can be a costly and time-consuming process. Regulatory hurdles and the varying approval processes across different countries can also create barriers to market entry and product adoption, leading to fragmented market access. Lastly, the declining global perception of the pandemic's urgency, as vaccination rates increase and severity decreases, could lead to a gradual decrease in overall demand, particularly from individual consumers.
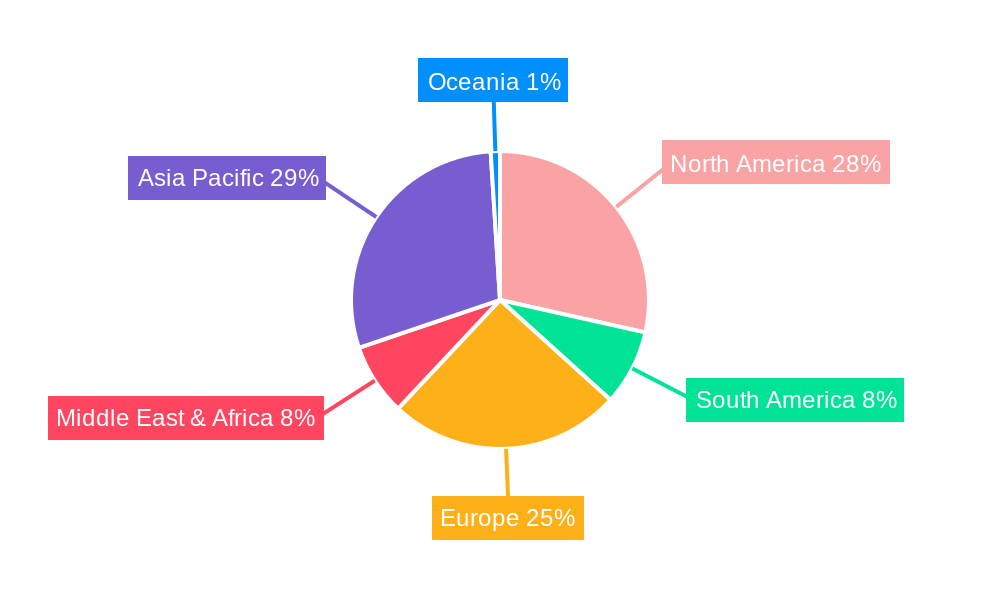
The Covid-19 Antigen Test Kits market is characterized by regional variations in demand, driven by public health policies, economic factors, and the prevalence of the virus. Among the various segments, Government Purchases are poised to be a dominant application, particularly within key regions like North America and Europe.
North America: This region, encompassing the United States and Canada, is anticipated to lead in terms of market share for government purchases of Covid-19 antigen test kits.
Europe: European nations, with their commitment to robust public health systems, are also significant drivers of government-led demand for antigen test kits.
While other regions like Asia-Pacific are also significant, the combined strategic focus on widespread testing infrastructure and proactive public health measures in North America and Europe, underpinned by substantial government funding, positions them as the dominant forces in the government purchase segment of the Covid-19 Antigen Test Kits market during the forecast period.
Several factors are poised to act as significant growth catalysts for the Covid-19 Antigen Test Kits industry. The continued integration of these kits into routine public health strategies, including school testing programs, workplace health monitoring, and travel screening, will ensure a steady demand. The development of more user-friendly, point-of-care, and at-home testing solutions will further expand accessibility and drive individual purchases. Furthermore, the potential for these kits to be adapted for the detection of other emerging infectious diseases, leveraging existing manufacturing infrastructure and technological advancements, presents a substantial long-term growth avenue. Innovations in multiplex testing capabilities, allowing for the simultaneous detection of multiple respiratory pathogens, will also enhance their utility and market appeal.
This comprehensive report on Covid-19 Antigen Test Kits provides an in-depth analysis of the market dynamics and future projections. It delves into the intricate interplay of various segments, including the differentiation between Very High Sensitivity (Clinical Sensitivity Greater than 80% PPA), High Sensitivity (Clinical Sensitivity Greater than 90% PPA), and Acceptable Sensitivity (Clinical Sensitivity Greater than 95% PPA) kits, and their respective market penetration. The report meticulously examines the application segments, highlighting the dominant role of Government Purchases and the growing significance of Individual Purchases, alongside emerging industrial applications. With a Study Period spanning from 2019 to 2033, a Base Year of 2025, and a detailed Forecast Period of 2025-2033, the report offers a robust outlook based on historical performance from 2019-2024. It identifies the key growth catalysts and challenges, providing strategic insights for stakeholders. The analysis also pinpoints dominant regions and countries driving market expansion and presents a comprehensive overview of leading players and their contributions to the industry.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Abbott, Alltest Biotech, Access Bio, Assure Tech, BIOHIT HealthCare, Innovation Scientific, Laihe Biotech, CTK Biotech, Well Biotech, Biotest Biotech, SD Biosensor, Testsea Biotechnology, Decheng Biotechnology, Beroni Group, .
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Covid-19 Antigen Test Kits," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Covid-19 Antigen Test Kits, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.