1. What is the projected Compound Annual Growth Rate (CAGR) of the Clinical Trial and Analysis Services?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Clinical Trial and Analysis Services
Clinical Trial and Analysis ServicesClinical Trial and Analysis Services by Type (/> On-site Services, Off-site Services), by Application (/> Hospital, Government & Regulatory Agencies, Pharmaceutical Company, Academic Centre, CRO, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
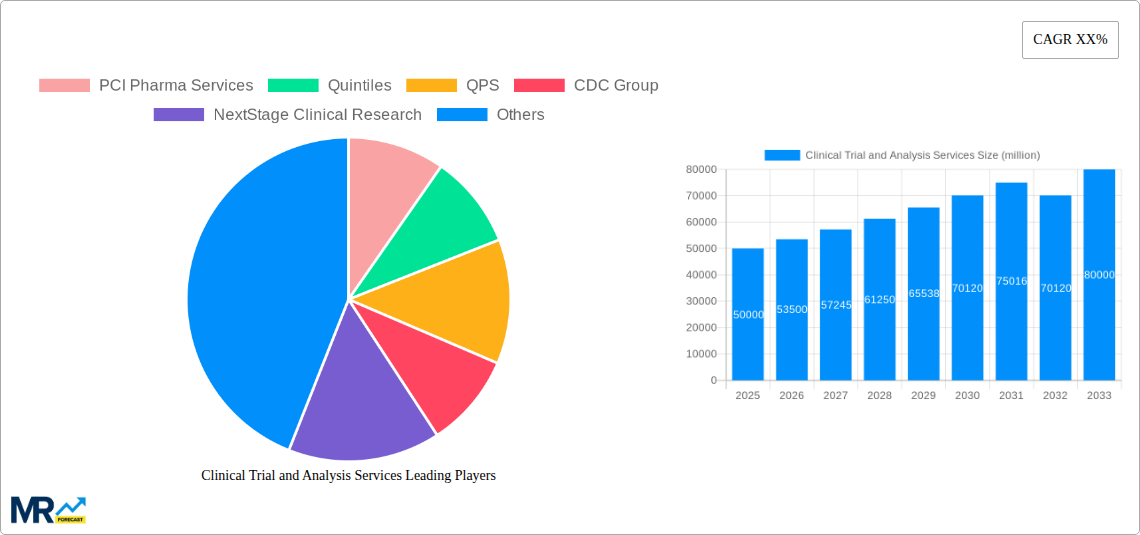
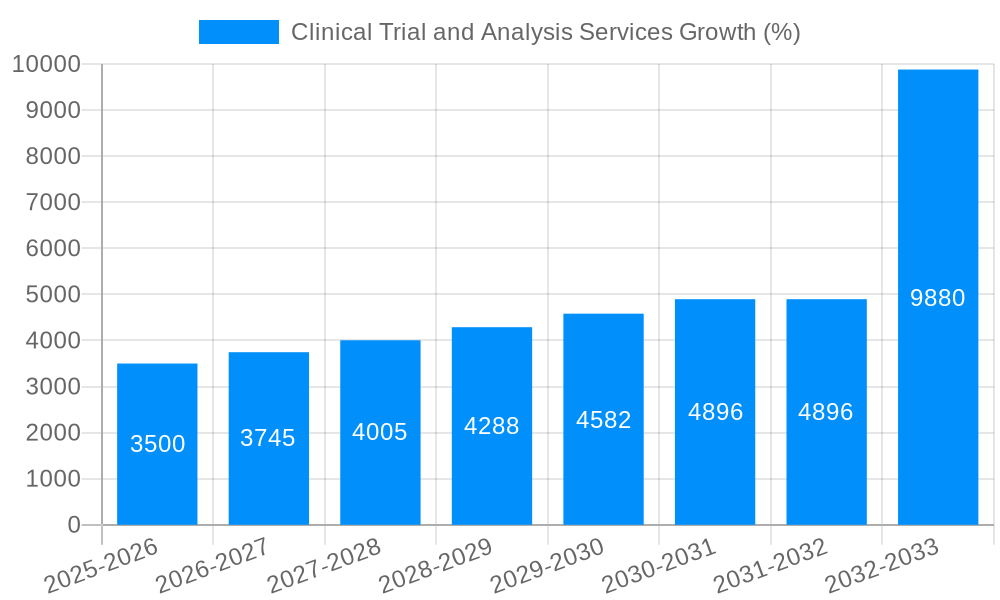
The global Clinical Trial and Analysis Services market is experiencing robust growth, driven by a surge in the number of clinical trials, increasing prevalence of chronic diseases, and the rising demand for advanced data analytics in drug development. The market, estimated at $50 billion in 2025, is projected to witness a Compound Annual Growth Rate (CAGR) of 7% from 2025 to 2033, reaching approximately $80 billion by 2033. This growth is fueled by technological advancements such as AI and machine learning, which enhance data analysis and accelerate trial timelines. Furthermore, the outsourcing trend amongst pharmaceutical and biotechnology companies is a significant contributor to market expansion, as they seek specialized expertise and cost-effective solutions from Contract Research Organizations (CROs). The market's segmentation encompasses various service types, including data management, biostatistical analysis, and regulatory consulting, each contributing to the overall growth trajectory. However, challenges such as stringent regulatory approvals, data privacy concerns, and the complexity of clinical trials pose potential restraints on market growth.
The competitive landscape is highly fragmented, with a mix of large multinational CROs like IQVIA and smaller specialized service providers. Key players are strategically investing in technological innovations and expanding their geographical reach to maintain a competitive edge. North America currently holds the largest market share, benefiting from a robust pharmaceutical industry and substantial investment in research and development. However, the Asia-Pacific region is expected to demonstrate rapid growth in the coming years due to rising healthcare expenditure and an increasing number of clinical trials conducted in emerging economies. The market's future trajectory will be shaped by the ongoing advancements in technology, regulatory landscape shifts, and the evolving needs of pharmaceutical and biotechnology companies. Continued focus on efficiency, data integrity, and patient safety will be crucial for market players to thrive in this dynamic sector.
The global clinical trial and analysis services market is experiencing robust growth, projected to reach multi-billion dollar valuations by 2033. The market's expansion is driven by several converging factors, including a burgeoning pharmaceutical and biotechnology industry, increasing prevalence of chronic diseases, and the consequent rise in demand for novel therapies. Technological advancements, such as the incorporation of AI and machine learning in data analysis, are significantly streamlining the clinical trial process, reducing timelines and costs. This trend has attracted substantial investments from both public and private sectors, further fueling market growth. The historical period (2019-2024) witnessed steady expansion, laying a strong foundation for the projected exponential growth during the forecast period (2025-2033). The estimated market size in 2025 is already in the billions, indicating a significant acceleration in market dynamics. Key market insights reveal a shift towards decentralized clinical trials (DCTs), leveraging telehealth and remote monitoring technologies to improve patient engagement and access to trials. This, coupled with a growing focus on real-world evidence (RWE) generation and utilization, is reshaping the industry landscape. The increasing complexity of clinical trials, particularly in areas like oncology and immunotherapy, necessitates specialized analytical services, boosting demand for advanced statistical techniques and data visualization tools. Regulatory changes globally also play a significant role, demanding greater transparency and data integrity throughout the clinical trial lifecycle. This trend is further shaping the need for robust and compliant clinical trial and analysis services. Competition within the market is fierce, with large multinational companies and specialized niche players vying for market share, driving innovation and service diversification.
Several factors contribute to the remarkable growth trajectory of the clinical trial and analysis services market. Firstly, the escalating global prevalence of chronic diseases like cancer, diabetes, and cardiovascular diseases necessitates a continuous pipeline of new therapies. This drives the demand for efficient and reliable clinical trial services to evaluate the safety and efficacy of these potential treatments. Secondly, technological advancements, particularly in areas like artificial intelligence (AI), machine learning (ML), and big data analytics, are revolutionizing clinical trial design, execution, and data analysis. These technologies help accelerate trial timelines, reduce costs, and improve the accuracy of results. Thirdly, an increased emphasis on patient-centricity is influencing the design and implementation of clinical trials. Decentralized clinical trials (DCTs), which utilize remote monitoring and telehealth technologies, are becoming increasingly popular, enabling broader patient participation and geographical reach. Moreover, the growing regulatory scrutiny and the need for robust data management and reporting systems are fueling the demand for specialized analytical services that ensure compliance and transparency. Finally, strategic partnerships and collaborations between pharmaceutical companies, contract research organizations (CROs), and technology providers are contributing to innovation and the development of more sophisticated and efficient solutions.
Despite the robust growth prospects, the clinical trial and analysis services market faces several challenges. The high cost associated with conducting clinical trials remains a major obstacle, particularly for smaller pharmaceutical companies and biotech startups. This expense encompasses not only the trial's operational aspects but also the intricate data analysis and regulatory compliance requirements. Another significant challenge is the complexity of regulations and guidelines governing clinical trials, which vary across different regions and therapeutic areas. Navigating this regulatory landscape necessitates significant expertise and resources, adding to the overall cost and complexity. Furthermore, the increasing demand for specialized skills and expertise in areas like biostatistics, data management, and regulatory affairs creates a talent shortage within the industry, impacting the capacity to handle the growing workload. Data privacy and security concerns are also paramount, particularly with the increasing use of electronic health records (EHRs) and other sensitive patient data. Ensuring compliance with data privacy regulations like GDPR adds another layer of complexity and cost to clinical trial operations. Lastly, competition within the market is intense, with numerous established players and emerging competitors vying for market share. This necessitates continuous innovation and the development of value-added services to differentiate and maintain competitiveness.
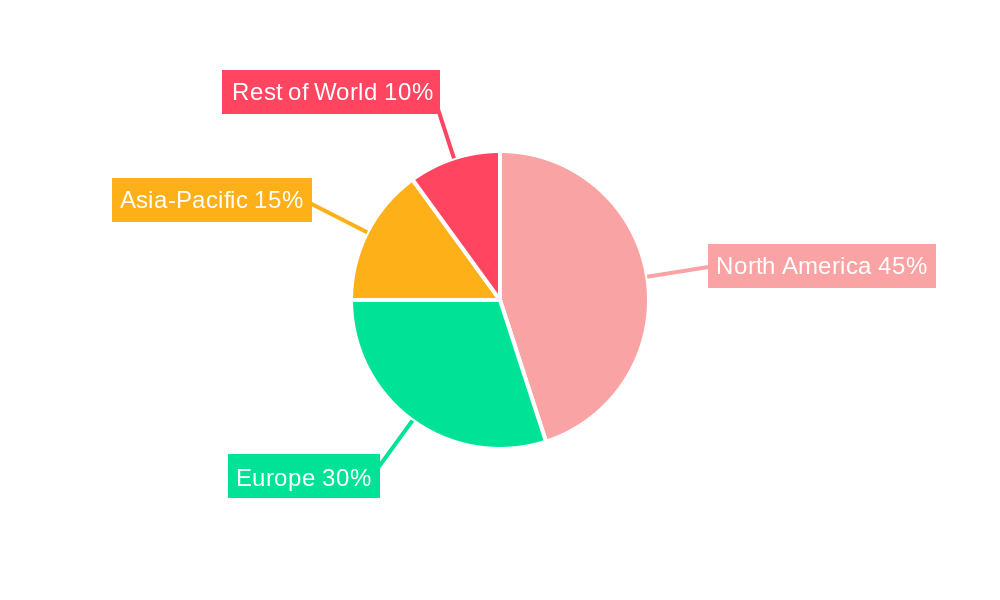
The North American market, particularly the United States, is expected to maintain a leading position in the global clinical trial and analysis services market due to the presence of major pharmaceutical companies, advanced research infrastructure, and substantial investment in biomedical research. However, other regions are rapidly gaining traction.
North America: High concentration of pharmaceutical companies, advanced infrastructure, and robust regulatory framework contribute to its dominance. The market here is expected to be in the billions.
Europe: A strong regulatory environment and the presence of leading CROs drive significant market growth, potentially in the hundreds of millions to billions.
Asia-Pacific: Rapidly growing healthcare sector, increasing investment in R&D, and expanding patient pool are key drivers, with projections into the hundreds of millions to billions.
Key Segments:
Oncology: The high prevalence of cancer and the ongoing development of targeted therapies contribute to this segment's robust growth. The market value here is likely to be hundreds of millions to billions.
Immunology: Similar to oncology, this segment benefits from increasing research activity and the rise in autoimmune diseases. Expected market value is similarly substantial.
The significant market value across these segments demonstrates the strong growth drivers and potential within the clinical trial and analysis services market. The projected market size for both regions and segments are projected to reach multi-billion dollar valuations within the forecast period.
The convergence of technological advancements, increased regulatory scrutiny, growing prevalence of chronic diseases, and a greater focus on patient-centricity are driving significant growth within the clinical trial and analysis services industry. These factors collectively create a fertile ground for innovation and expansion, resulting in a rapidly evolving market with substantial growth potential.
This report provides a comprehensive overview of the global clinical trial and analysis services market, encompassing market size estimations, segment analysis, regional breakdowns, and competitive landscapes for the period 2019-2033. It identifies key growth drivers, challenges, and opportunities, offering valuable insights for stakeholders in the pharmaceutical, biotechnology, and clinical research industries. The report analyzes the impact of technological advancements and regulatory changes on the market, providing actionable intelligence for strategic decision-making.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include PCI Pharma Services, Quintiles, QPS, CDC Group, NextStage Clinical Research, SanaClis, Caidya, IQVIA, WuXi AppTec, CIRS, YIDU Technology, Precision Scientific, EPS TIGERMED (SUZHOU)CO..LTD, BOSTAL, JOINN, YUANBO, ZC-MED, George Clinical, ClinHope, PHARMARON, Taicheng Yaozhong, Thermo Fisher Scientific, .
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Clinical Trial and Analysis Services," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Clinical Trial and Analysis Services, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.