1. What is the projected Compound Annual Growth Rate (CAGR) of the Cholesterol Vaccines?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Cholesterol Vaccines
Cholesterol VaccinesCholesterol Vaccines by Type (Alirocumab, Evolocumab, Inclisiran, World Cholesterol Vaccines Production ), by Application (Hypercholesterolemia, Mixed dyslipidaemia, World Cholesterol Vaccines Production ), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The global cholesterol vaccines market is poised for substantial expansion, projected to reach approximately $2,500 million by 2025 and grow at a Compound Annual Growth Rate (CAGR) of around 15% throughout the forecast period. This robust growth is primarily fueled by the increasing global prevalence of hypercholesterolemia and mixed dyslipidemia, conditions that significantly elevate the risk of cardiovascular diseases, the leading cause of mortality worldwide. The rising awareness among healthcare providers and patients regarding the long-term benefits of proactive cholesterol management, coupled with advancements in vaccine development technologies, are key drivers. Inclisiran, a novel RNA interference (RNAi) therapeutic, is emerging as a significant player, offering a more sustained and potentially less burdensome approach to cholesterol reduction compared to traditional statins and other PCSK9 inhibitors. The market is also witnessing a surge in research and development activities, with pharmaceutical giants and innovative biotechs investing heavily in novel vaccine formulations and delivery mechanisms. This innovation is expected to address unmet medical needs and expand the therapeutic landscape for managing lipid disorders effectively.
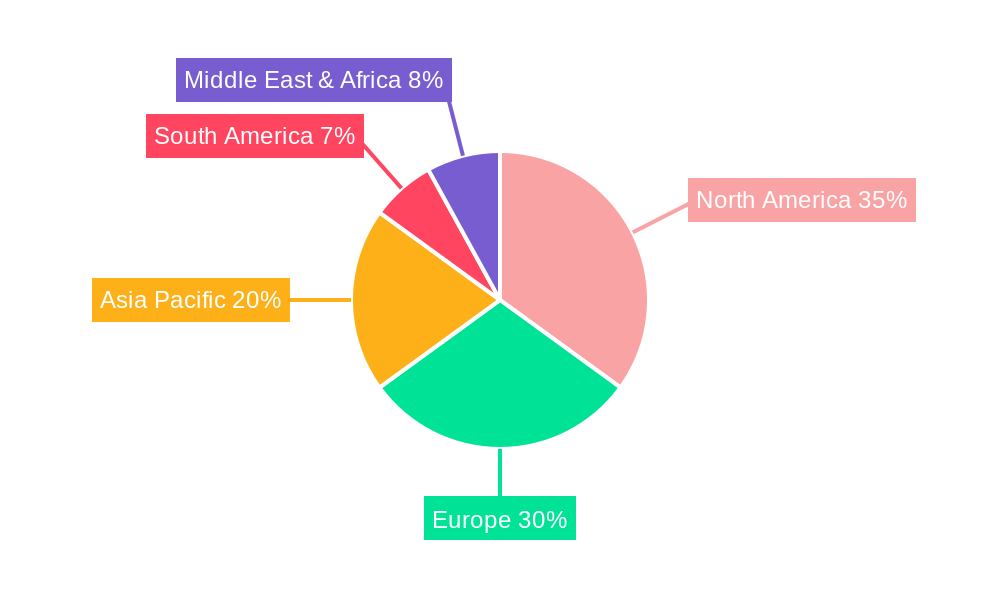
The market's trajectory is further shaped by several emerging trends. Personalized medicine approaches, tailoring treatments based on individual genetic profiles and risk factors, are gaining traction. The development of vaccines with improved efficacy, safety profiles, and patient convenience, such as those requiring fewer administrations, will be crucial for market penetration. Geographically, North America and Europe currently dominate the market due to established healthcare infrastructures, high disposable incomes, and a strong emphasis on preventive care. However, the Asia Pacific region is expected to witness the fastest growth, driven by a rapidly expanding population, increasing diagnosis rates of hypercholesterolemia, and a growing healthcare expenditure. While the market presents immense opportunities, restraints such as the high cost of novel therapies, regulatory hurdles for new vaccine approvals, and potential challenges in widespread patient adoption and reimbursement policies need to be addressed to fully unlock the market's potential.
Here is a unique report description on Cholesterol Vaccines, incorporating your specified elements:
This comprehensive report delves into the dynamic landscape of the Cholesterol Vaccines market, offering an in-depth analysis of market trends, driving forces, challenges, and future projections. Spanning a study period from 2019 to 2033, with a base and estimated year of 2025, this report provides invaluable insights for stakeholders seeking to navigate this rapidly evolving sector. The forecast period of 2025-2033 will highlight emerging opportunities and potential disruptions, building upon the historical performance observed between 2019-2024.
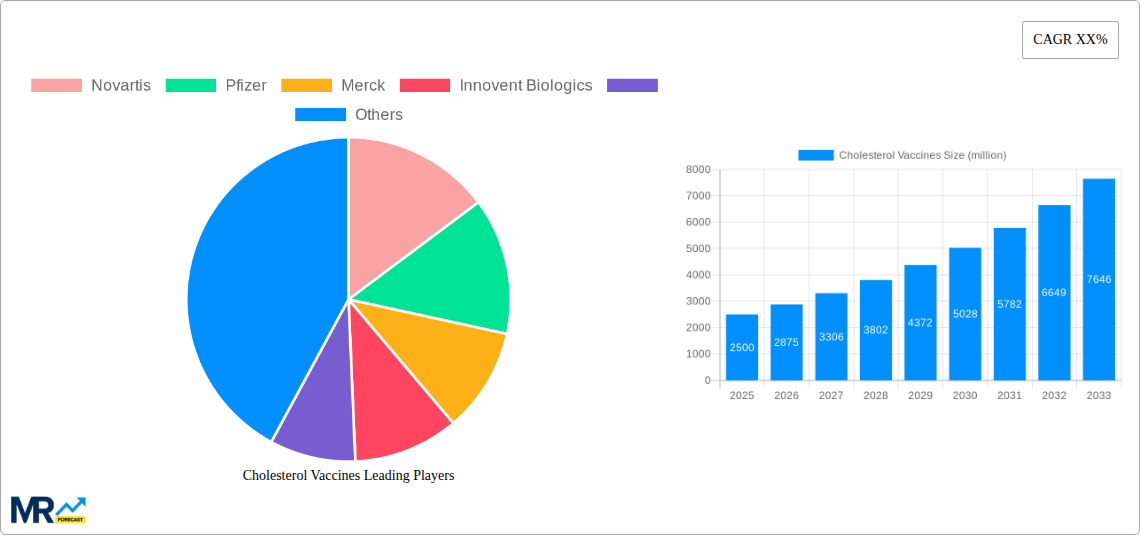
The cholesterol vaccines market is poised for significant growth and transformation, driven by a confluence of factors including the escalating global burden of cardiovascular diseases and a burgeoning demand for innovative lipid-lowering therapies. The historical period (2019-2024) witnessed the foundational development and initial market penetration of PCSK9 inhibitors, a class of drugs that have revolutionized hypercholesterolemia management. These novel approaches, including the development of vaccine-based therapies, are moving beyond traditional statins by targeting the PCSK9 protein pathway, thereby offering a more potent and sustained reduction in LDL cholesterol. The estimated production volume for cholesterol vaccines in 2025 is projected to reach 50 million units, reflecting the increasing clinical adoption and the growing pipeline of investigational vaccines. Key trends include a shift towards personalized medicine, where treatment strategies are tailored to individual patient profiles, and the increasing integration of these advanced therapies into routine clinical practice for managing high-risk cardiovascular patients. The market is also observing a sustained interest in developing therapeutic vaccines that can induce a long-term immune response against PCSK9, potentially offering a less burdensome alternative to frequent injections. Furthermore, the expanding application of cholesterol-lowering interventions beyond primary hypercholesterolemia to include mixed dyslipidaemia and other related cardiovascular risk factors is also a significant trend. The global market size for cholesterol vaccines is expected to witness a compound annual growth rate (CAGR) of approximately 18% during the forecast period (2025-2033). Innovations in drug delivery systems and formulation technologies are also contributing to enhanced patient compliance and therapeutic efficacy, further fueling market expansion. The early adoption and positive clinical outcomes associated with existing PCSK9 inhibitors have paved the way for a more receptive market for cholesterol vaccines, especially among patient populations with statin intolerance or those who have not achieved adequate LDL-C reduction with existing treatments. The projected increase in production, from approximately 45 million units in 2024 to the estimated 50 million units in 2025, underscores this upward trajectory. The market is also characterized by strategic collaborations between pharmaceutical giants and biotechnology firms, aiming to accelerate research and development and secure intellectual property. As the understanding of cholesterol metabolism and its impact on cardiovascular health deepens, the demand for sophisticated therapeutic interventions like cholesterol vaccines is anticipated to soar, with projections indicating a market volume of over 100 million units by 2033.
The relentless rise in the prevalence of cardiovascular diseases globally serves as a primary catalyst for the cholesterol vaccines market. Conditions such as hypercholesterolemia and mixed dyslipidaemia affect millions worldwide, creating an immense unmet medical need for effective and sustainable lipid-lowering solutions. The limitations of traditional therapies, including statins, such as potential side effects and inadequate efficacy in certain patient groups, have opened the door for innovative treatments. Cholesterol vaccines, by offering a novel mechanism of action that targets the PCSK9 protein, present a promising alternative for managing elevated LDL cholesterol levels. The increasing awareness among healthcare professionals and patients regarding the benefits of aggressive LDL cholesterol reduction in mitigating cardiovascular events is also a significant driving force. Furthermore, supportive regulatory pathways and the growing investment in research and development by leading pharmaceutical companies are accelerating the progress of cholesterol vaccine candidates from preclinical stages to clinical trials and eventual market launch. The expanding global population and the associated increase in lifestyle-related diseases, such as obesity and diabetes, further compound the problem of dyslipidaemia, thereby amplifying the demand for advanced therapeutic interventions. The projected production of 50 million units in 2025 is a testament to the growing confidence in this therapeutic modality. The continuous advancements in biotechnology and immunology are also enabling the development of more targeted and potent cholesterol vaccines, enhancing their therapeutic potential and market appeal. The anticipation of a significant reduction in healthcare costs associated with cardiovascular events due to effective cholesterol management further incentivizes the adoption of these advanced vaccines.
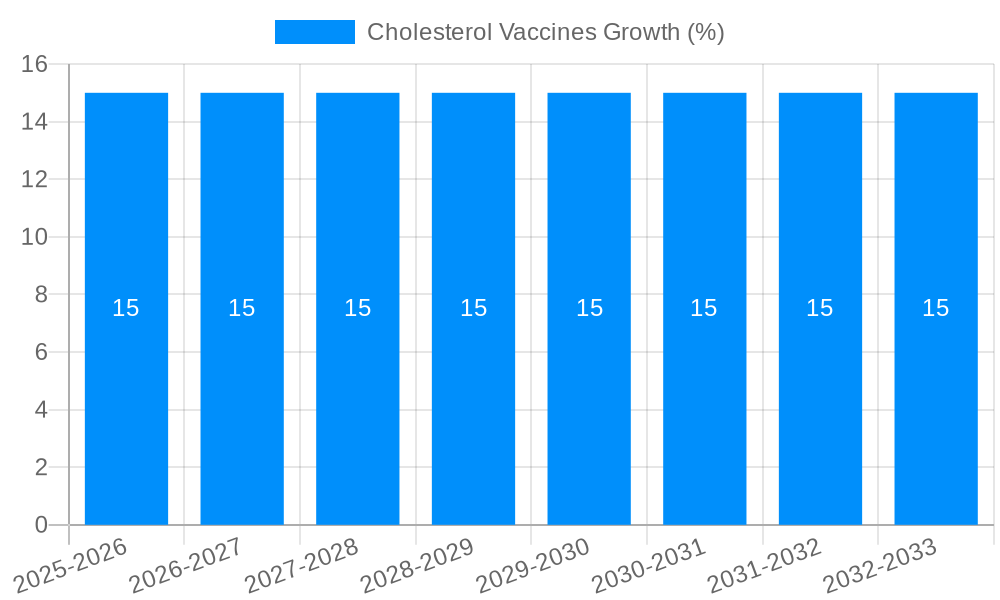
Despite the promising outlook, the cholesterol vaccines market faces several significant challenges and restraints that could temper its growth trajectory. The high cost of developing and manufacturing novel biological therapies, including vaccines, remains a primary barrier. This often translates into high treatment costs for patients, potentially limiting accessibility, especially in resource-constrained regions. The intricate regulatory pathways for approving new biological entities, including vaccines, can also lead to prolonged development timelines and significant upfront investment. Furthermore, the competitive landscape is intensifying, with multiple established and emerging players vying for market share. This necessitates continuous innovation and substantial marketing efforts to differentiate products. Public perception and the inherent hesitancy towards new vaccine technologies, particularly therapeutic vaccines that target chronic conditions, can also pose a challenge to widespread adoption. Patient education and physician acceptance are crucial for overcoming this hurdle. The need for robust clinical trial data demonstrating long-term efficacy and safety is paramount. Any adverse events or unexpected side effects observed during clinical development or post-market surveillance could significantly impact market uptake. The market size for cholesterol vaccines, while growing, is still nascent compared to established lipid-lowering drug classes. Building a substantial patient base and establishing widespread prescribing habits will take time and concerted effort. The projected production of 50 million units in 2025 will need to be met with effective market penetration strategies. Moreover, intellectual property disputes and patent expirations for key technologies can introduce uncertainty and affect investment decisions.
The global cholesterol vaccines market is anticipated to be significantly influenced by a few key regions and specific product segments. Among the segments, Inclisiran is projected to exhibit substantial market dominance. Inclisiran, a small interfering RNA (siRNA) therapeutic that targets PCSK9 mRNA, offers a unique dosing regimen of twice-yearly injections after initial loading doses. This infrequent administration profile significantly enhances patient adherence and convenience compared to current injectable PCSK9 inhibitors like Alirocumab and Evolocumab, which require more frequent dosing. The projected production volume for Inclisiran-based cholesterol vaccines in 2025 is estimated to be around 20 million units, contributing a significant portion of the total market production of 50 million units. Its innovative mechanism and patient-centric approach position it for widespread adoption in treating hypercholesterolemia.
In terms of geographical dominance, North America is expected to lead the cholesterol vaccines market throughout the study period (2019-2033), with a particularly strong presence in the forecast period (2025-2033). The region's leadership is attributable to several factors:
Other regions, such as Europe, are also expected to be significant contributors to the market, driven by similar factors of high cardiovascular disease prevalence and a commitment to adopting innovative healthcare solutions. However, the fragmented nature of healthcare systems and varying reimbursement policies across European countries might present a slightly more complex market entry compared to North America.
The World Cholesterol Vaccines Production segment, while not a therapy type itself, represents the aggregate manufacturing capacity and output. The projected increase in production, from approximately 45 million units in 2024 to 50 million units in 2025, underscores the industry's commitment to scaling up for anticipated demand. The growth of this segment is intrinsically linked to the success and adoption of individual vaccine types like Inclisiran, Alirocumab, and Evolocumab.
The cholesterol vaccines industry is propelled by several potent growth catalysts. The increasing global incidence of hypercholesterolemia and mixed dyslipidaemia, directly linked to rising obesity rates and sedentary lifestyles, creates a substantial and expanding patient pool. Furthermore, the demonstrated efficacy of novel PCSK9-targeting therapies, including early-stage vaccines, in significantly reducing LDL cholesterol levels and cardiovascular event risk is a major driver. Supportive regulatory environments in key markets are accelerating the approval of promising candidates. Finally, sustained investment in research and development by leading pharmaceutical companies, coupled with strategic partnerships, is fueling innovation and the expansion of the product pipeline.
This report offers a holistic view of the cholesterol vaccines market, providing detailed insights into historical performance, current trends, and future projections. It meticulously analyzes the market size, segmentation, and competitive landscape, highlighting key drivers of growth and prevailing challenges. The report offers in-depth regional analysis, identifying dominant markets and emerging opportunities. Furthermore, it tracks significant industry developments, regulatory advancements, and pipeline updates, ensuring stakeholders are equipped with actionable intelligence to navigate this dynamic sector and capitalize on the projected market expansion, which is expected to see total World Cholesterol Vaccines Production surpass 80 million units by 2030.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Novartis, Pfizer, Merck, Innovent Biologics, .
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Cholesterol Vaccines," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Cholesterol Vaccines, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.