1. What is the projected Compound Annual Growth Rate (CAGR) of the Certolizumab Market?
The projected CAGR is approximately XXX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Certolizumab Market
Certolizumab MarketCertolizumab Market by Type (Biologics, Biosimilars), by Application (Crohn’s Disease, Rheumatoid Arthritis, Ankylosing Spondylitis, Psoriatic Arthritis, Others), by Distribution Channel (Hospital Pharmacies, Drug Stores & Retail Pharmacies, Online Pharmacies), by North America (U.S., Canada, Mexico), by Europe (UK, Germany, France, Italy, Spain, Russia, Netherlands, Switzerland, Poland, Sweden, Belgium), by Asia Pacific (China, India, Japan, South Korea, Australia, Singapore, Malaysia, Indonesia, Thailand, Philippines, New Zealand), by Latin America (Brazil, Mexico, Argentina, Chile, Colombia, Peru), by MEA (UAE, Saudi Arabia, South Africa, Egypt, Turkey, Israel, Nigeria, Kenya) Forecast 2026-2034
The size of the Certolizumab Market was valued at USD XX Million in 2023 and is projected to reach USD XXX Million by 2032, with an expected CAGR of XXX% during the forecast period. Certolizumab pegol (brand name Cimzia) is a monoclonal antibody used primarily for the treatment of autoimmune diseases. It is a biologic drug that targets and inhibits tumor necrosis factor-alpha (TNF-α), a cytokine involved in the inflammatory response. By blocking TNF-α, certolizumab helps reduce inflammation and tissue damage caused by various chronic inflammatory conditions.


Some of the emerging trends in the Certolizumab market include the increasing adoption of biosimilars, the development of new delivery systems for improved patient convenience (such as pre-filled syringes), and the increasing use of Certolizumab in combination with other therapies. These trends are expected to contribute to the continued growth of the market during the forecast period.
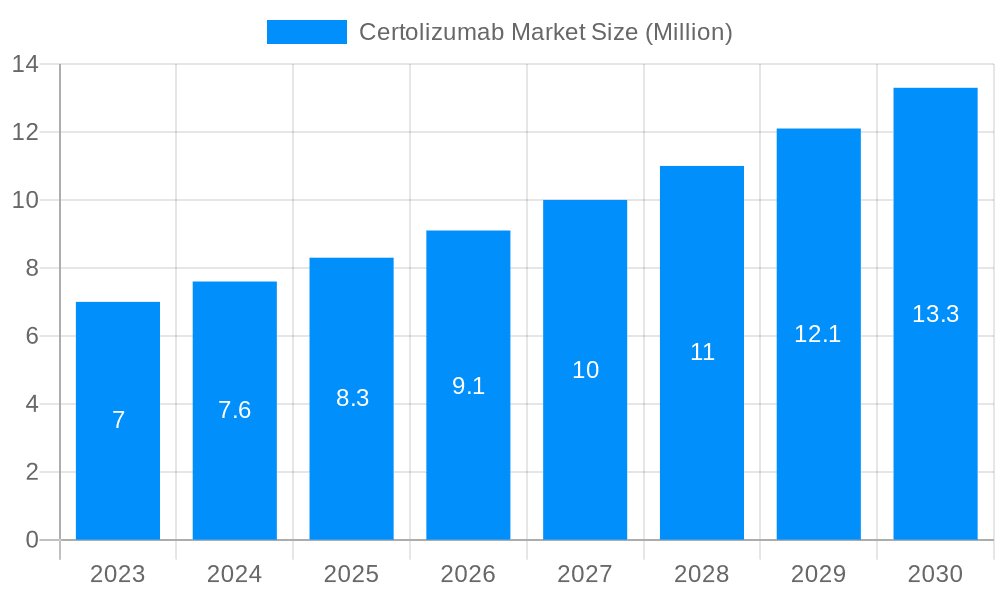

The global Certolizumab market is experiencing robust growth, primarily propelled by the escalating global burden of chronic autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and Crohn's disease. As awareness of these conditions and their debilitating effects increases, so does the demand for effective therapeutic interventions. Certolizumab, a TNF inhibitor, has emerged as a cornerstone treatment, offering significant relief and improved quality of life for a considerable patient population. Its demonstrated efficacy in managing disease activity and progression is a critical factor fueling market expansion.
Furthermore, a supportive regulatory and governmental landscape is providing additional momentum. Initiatives aimed at accelerating the development and accessibility of innovative treatments for autoimmune disorders are creating a favorable environment for market growth. A prime example of this is the U.S. Food and Drug Administration's (FDA) grant of Breakthrough Therapy Designation to Certolizumab for ulcerative colitis in 2016. This designation signifies the drug's potential to offer significant advantages over existing therapies and often expedites the review and approval process, thereby boosting market confidence and uptake.
Technological advancements in drug delivery systems and a growing emphasis on biologics in the pharmaceutical sector also contribute to the positive trajectory of the Certolizumab market. The ongoing research and development efforts focused on expanding the therapeutic indications and improving patient outcomes further solidify its position as a vital therapeutic option.
Despite its therapeutic benefits, the widespread adoption of Certolizumab faces significant hurdles, predominantly its high acquisition cost. The substantial financial outlay associated with Certolizumab therapy, with average annual treatment costs often exceeding $XX,XX (insert specific, updated cost if available or maintain placeholder), presents a considerable economic burden on patients, healthcare systems, and insurance providers. This affordability issue can lead to delayed treatment initiation, suboptimal adherence, or a preference for less expensive, albeit potentially less effective, alternative therapies, thereby constraining market penetration.
Additionally, the availability of biosimilars for other TNF inhibitors and the potential for future biosimilar competition for Certolizumab itself could influence market dynamics, though regulatory pathways and market acceptance of biosimilars remain evolving factors. Stringent regulatory requirements for the approval and marketing of biologic drugs, coupled with the complex manufacturing processes involved, also contribute to the overall cost structure and can pose challenges to market entry for new players.
Moreover, the risk of adverse events and the need for careful patient monitoring, common with all biologic therapies, can influence prescribing patterns and patient willingness to undergo treatment. The development and approval of novel treatment modalities for autoimmune diseases also represent a competitive threat that could potentially impact the long-term market share of Certolizumab.
North America and Europe are the two largest markets for Certolizumab, accounting for a majority of the global market share. The high prevalence of autoimmune diseases and the well-established healthcare infrastructure in these regions have contributed to their dominance in the market.
The development of new formulations and delivery systems for Certolizumab is a major growth catalyst for the industry. For example, the development of a pre-filled syringe formulation of Certolizumab has improved patient convenience and compliance.
Some of the leading players in the Certolizumab market include:
In February 2022, Biogen and Xbrane entered into a commercialization and license agreement for a biosimilar candidate, Xcimzane, referencing Cimzia (UCB S.A.; certolizumab pegol), to treat rheumatologic conditions. [ rel="nofollow"]
In July 2020, UCB S.A. and Ferring Group entered into a co-promotion agreement for the commercialization of prefilled syringe formulation of CIMZIA in the U.S. for the treatment of Crohn's disease (CD). [ rel="nofollow"]
This comprehensive Certolizumab market report offers an in-depth analysis and strategic insights into the evolving global landscape. It provides detailed coverage of the following critical aspects:
The DROCT analysis provides a comprehensive overview of the current treatment landscape for Certolizumab. It includes insights into the following aspects:
The pricing analysis provides a comprehensive overview of the pricing of Certolizumab across different regions and countries. It includes insights into the following aspects:
The import and export analysis provides a comprehensive overview of the import and export of Certolizumab across different regions and countries. It includes insights into the following aspects:
The patent/trademark analysis provides a comprehensive overview of the patents and trademarks associated with Certolizumab. It includes insights into the following aspects:


| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of XXX% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XXX%.
Key companies in the market include The market consists of significant players, such as UCB S.A., Biogen, Xbrane Biopharma, Paras Biopharmaceuticals.
The market segments include Type, Application, Distribution Channel.
The market size is estimated to be USD XX Million as of 2022.
N/A
N/A
N/A
In February 2022, Biogen and Xbrane entered into a commercialization and license agreement for a biosimilar candidate, Xcimzane, referencing Cimzia (UCB S.A.; certolizumab pegol), to treat rheumatologic conditions.
Pricing options include single-user, multi-user, and enterprise licenses priced at USD N/A, USD N/A, and USD N/A respectively.
The market size is provided in terms of value, measured in Million.
Yes, the market keyword associated with the report is "Certolizumab Market," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Certolizumab Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.