1. What is the projected Compound Annual Growth Rate (CAGR) of the Cephalosporin APIs and Cephalosporin Intermediates?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Cephalosporin APIs and Cephalosporin Intermediates
Cephalosporin APIs and Cephalosporin IntermediatesCephalosporin APIs and Cephalosporin Intermediates by Type (Cephalosporin APIs, Cephalosporin Intermediates), by Application (Injections, Oral Preparations), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The global market for Cephalosporin APIs and Intermediates is poised for robust expansion, driven by the persistent demand for effective antibacterial treatments and the ongoing development of new cephalosporin derivatives. The market size is estimated to be around $10,500 million in 2025, with a projected Compound Annual Growth Rate (CAGR) of approximately 8.5% from 2025 to 2033. This growth is primarily fueled by the increasing prevalence of bacterial infections worldwide, coupled with rising healthcare expenditures and a growing emphasis on the production of high-quality, cost-effective pharmaceutical ingredients. Key therapeutic areas, including respiratory tract infections, urinary tract infections, and skin and soft tissue infections, continue to rely heavily on cephalosporins, underscoring their critical role in modern medicine. Furthermore, advancements in manufacturing technologies and the continuous exploration of novel cephalosporin formulations are expected to sustain market momentum. The market's value, projected to reach $20,200 million by 2033, reflects a dynamic landscape shaped by both established players and emerging manufacturers vying for market share through product innovation and strategic partnerships.
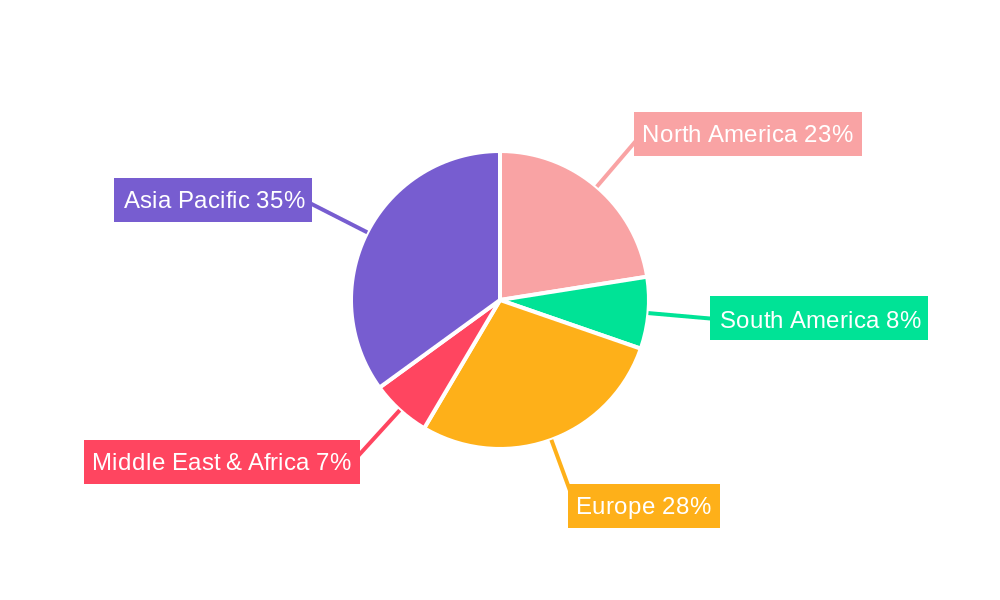
The market is segmented into Cephalosporin APIs and Cephalosporin Intermediates, with APIs holding a larger share due to direct application in finished drug formulations. Applications span both Injections and Oral Preparations, with injectables often commanding higher value owing to their efficacy in treating severe infections. Geographically, the Asia Pacific region, particularly China and India, is emerging as a significant manufacturing hub and a growing consumer market due to a large population and increasing access to healthcare. North America and Europe remain dominant markets due to well-established healthcare infrastructure and high demand for advanced pharmaceutical products. However, restraints such as the increasing emergence of antibiotic resistance, stringent regulatory hurdles for new drug approvals, and the availability of alternative treatments pose challenges. Despite these, the consistent need for reliable and effective antibiotics ensures a sustained demand for cephalosporin-based products, making this market a vital segment within the global pharmaceutical industry.
Here's a comprehensive report description on Cephalosporin APIs and Cephalosporin Intermediates, incorporating your specified elements and word counts.
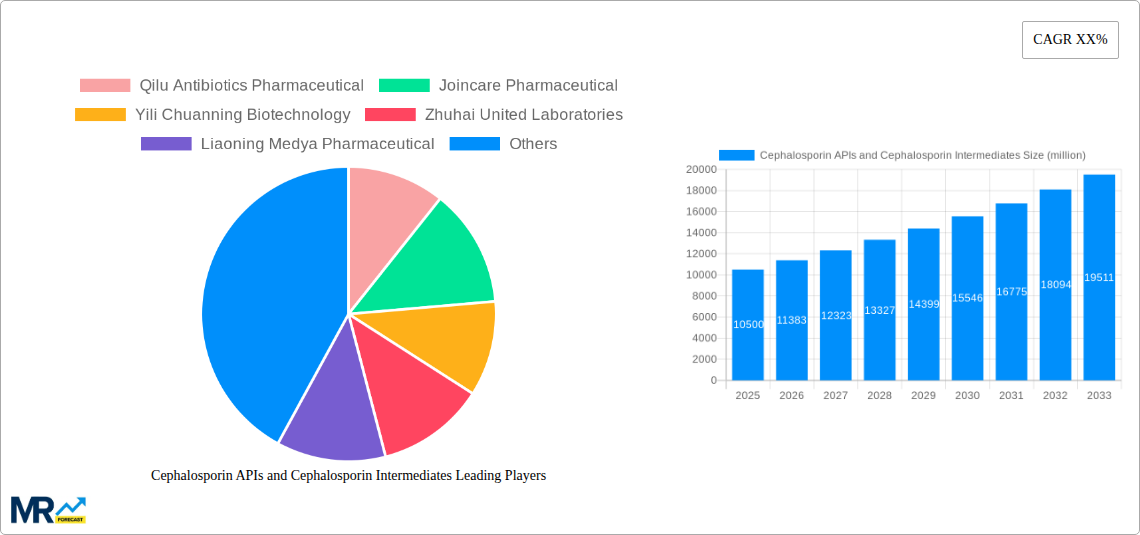
The global market for Cephalosporin APIs (Active Pharmaceutical Ingredients) and Cephalosporin Intermediates is poised for substantial growth, driven by an escalating demand for effective antibacterial treatments and a robust pipeline of new product developments. The study period, spanning from 2019 to 2033, with a base year of 2025 and a forecast period of 2025-2033, encapsulates a dynamic market landscape. Historical data from 2019-2024 indicates a steady upward trajectory, with significant investments in research and development fueling innovation. XXX indicates key market insights, highlighting the persistent prevalence of bacterial infections, particularly in developing economies, as a primary demand driver. The increasing incidence of antibiotic resistance is also creating an urgent need for novel and potent cephalosporin derivatives, thus stimulating the market for both APIs and intermediates. The market is segmented by Type into Cephalosporin APIs and Cephalosporin Intermediates, and by Application into Injections and Oral Preparations. The dominance of injectable formulations, due to their faster onset of action and higher bioavailability, is expected to continue. However, the convenience and patient compliance associated with oral preparations are also contributing to their growing market share. Industry Developments, a crucial segment, reflects the continuous efforts of manufacturers to optimize production processes, ensure product quality, and meet stringent regulatory standards. The market's value is anticipated to reach several hundred million units, with significant contributions from both established and emerging players. Geographic shifts in manufacturing capabilities and increasing collaborations between API manufacturers and formulation companies are also shaping the market's future. The focus on developing next-generation cephalosporins with broader spectrum activity and improved pharmacokinetic profiles will be a key determinant of market expansion throughout the forecast period.
The market for Cephalosporin APIs and Cephalosporin Intermediates is propelled by a confluence of powerful factors. Foremost among these is the persistent and widespread burden of bacterial infections globally. Despite advancements in medical science, bacterial pathogens continue to pose a significant threat to public health, necessitating a constant supply of effective antimicrobial agents. Cephalosporins, with their broad spectrum of activity and established safety profiles, remain a cornerstone of antibiotic therapy for a wide range of infections. Furthermore, the escalating global crisis of antimicrobial resistance (AMR) is creating an urgent and unmet medical need. As bacteria evolve and develop resistance to existing drugs, there is a renewed focus on developing and producing novel cephalosporin derivatives with enhanced efficacy against resistant strains. This imperative drives innovation in both the discovery of new APIs and the efficient synthesis of complex intermediates required for their production. The aging global population also contributes to market growth, as older individuals are generally more susceptible to infections. Moreover, increased access to healthcare in emerging economies, coupled with rising disposable incomes, is leading to greater demand for pharmaceutical products, including cephalosporins. Pharmaceutical companies are also actively investing in the research and development of new cephalosporin formulations and combinations, which in turn stimulates demand for their constituent APIs and intermediates.
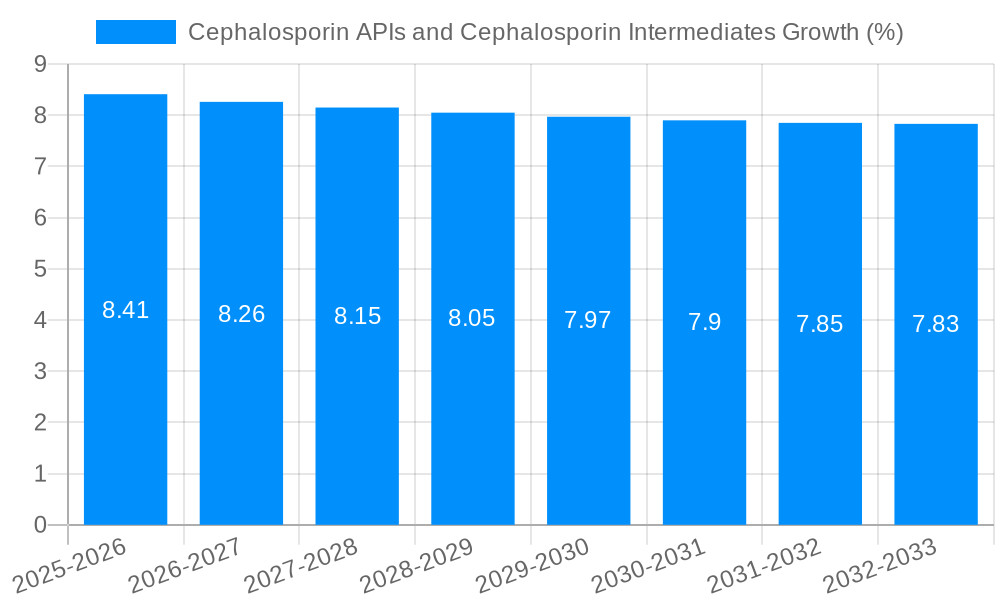
Despite the positive market outlook, the Cephalosporin APIs and Cephalosporin Intermediates sector faces several significant challenges and restraints. One of the most pressing concerns is the growing threat of antimicrobial resistance (AMR). The widespread use and misuse of antibiotics have led to the emergence of highly resistant bacterial strains, which limits the effectiveness of many existing cephalosporin drugs. This necessitates constant innovation and investment in developing new, more potent cephalosporins, which can be a time-consuming and expensive process. Furthermore, stringent regulatory requirements and the lengthy approval processes for new drug substances and manufacturing facilities can hinder market entry and expansion, especially for smaller players. The complex supply chains involved in the production of cephalosporin APIs and intermediates, often spanning multiple countries, can be susceptible to disruptions due to geopolitical instability, trade disputes, or unforeseen global events, impacting availability and pricing. Price erosion due to intense competition, particularly from generic manufacturers in emerging markets, can also put pressure on profit margins for API producers. Environmental concerns related to pharmaceutical manufacturing, including waste management and the potential for antibiotic residues in the environment, are also leading to stricter regulations and increased compliance costs. Finally, the development of alternative therapeutic approaches, such as phage therapy or novel non-antibiotic antimicrobial agents, could potentially challenge the long-term dominance of antibiotics like cephalosporins, though these are still in early stages of widespread adoption.
The Cephalosporin Intermediates segment is projected to exhibit robust growth and potentially dominate the market, particularly driven by the manufacturing capabilities and cost-effectiveness prevalent in Asia-Pacific, with a special emphasis on China and India. This dominance stems from several interconnected factors.
While Cephalosporin APIs also represent a significant market, the intermediates segment often precedes and underpins API production. The sheer volume and diversity of intermediates required for the various generations and types of cephalosporins, coupled with the cost sensitivity associated with their production, strongly favor regions with established manufacturing prowess. The competitive landscape for intermediates is characterized by a larger number of players, each specializing in specific chemical pathways, further contributing to their market share. This intricate interplay of manufacturing efficiency, specialized skills, and supportive infrastructure positions the Cephalosporin Intermediates segment, especially within the Asia-Pacific region, as a key driver of market growth and dominance.
The growth of the Cephalosporin APIs and Cephalosporin Intermediates industry is catalyzed by an increasing global demand for antibiotics, driven by the persistent prevalence of bacterial infections and the alarming rise of antimicrobial resistance. Continuous investment in R&D for novel cephalosporin derivatives with enhanced efficacy and broader spectrum activity is a key catalyst. Furthermore, the expanding healthcare infrastructure and rising disposable incomes in emerging economies are fueling greater accessibility and consumption of pharmaceutical products, including cephalosporins. Industry developments focused on process optimization, cost reduction, and stringent quality control also act as significant growth enablers, ensuring a steady supply of high-quality APIs and intermediates to meet global pharmaceutical needs.
This report offers a comprehensive examination of the global Cephalosporin APIs and Cephalosporin Intermediates market, providing in-depth analysis across its entire value chain. It delves into market dynamics, including historical trends from 2019-2024, current market size (base year 2025), and future projections for the forecast period of 2025-2033. The report meticulously segments the market by type (APIs and Intermediates) and application (Injections and Oral Preparations), offering granular insights into the performance of each category. It also scrutinizes the driving forces, challenges, and growth catalysts shaping the industry. Furthermore, the report identifies and profiles key regional markets and leading players, alongside significant industry developments and strategic initiatives. This comprehensive coverage ensures stakeholders have the necessary information to make informed strategic decisions in this vital pharmaceutical sector.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Qilu Antibiotics Pharmaceutical, Joincare Pharmaceutical, Yili Chuanning Biotechnology, Zhuhai United Laboratories, Liaoning Medya Pharmaceutical, Henan Kangda Pharmaceutical, North China Pharmaceutical, Cspc Holdings, Wichita, Shandong Jincheng Pharmaceutical, Harbin Pharmaceutical, Zhejiang Huafang Pharmacertical, Sichuan Kelun Pharmaceutical, Lizhu Group, Zhejiang Dongying Pharmaceutical, Shandong Lukang Pharmaceutical, Zhejiang Anglikang Pharmaceutical, Zhejiang East-Asia Pharmaceutical, Hebei Hejia Pharmaceutical, GSK, CordenPharma, Dragon Pharma, Orchid Pharma, Aurobindo, ACS Dobfar SpA, Novartis, Nectar Lifesciences, Jeil Pharmaceutical, Orchid Pharma, Aurobindo, .
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Cephalosporin APIs and Cephalosporin Intermediates," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Cephalosporin APIs and Cephalosporin Intermediates, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.