1. What is the projected Compound Annual Growth Rate (CAGR) of the Cell-bridging Bispecific Antibodies?
The projected CAGR is approximately 39.0%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Cell-bridging Bispecific Antibodies
Cell-bridging Bispecific AntibodiesCell-bridging Bispecific Antibodies by Type (T Cell Engagers, NK Cell Engagers), by Application (Hematological Cancers, Solid Tumors), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
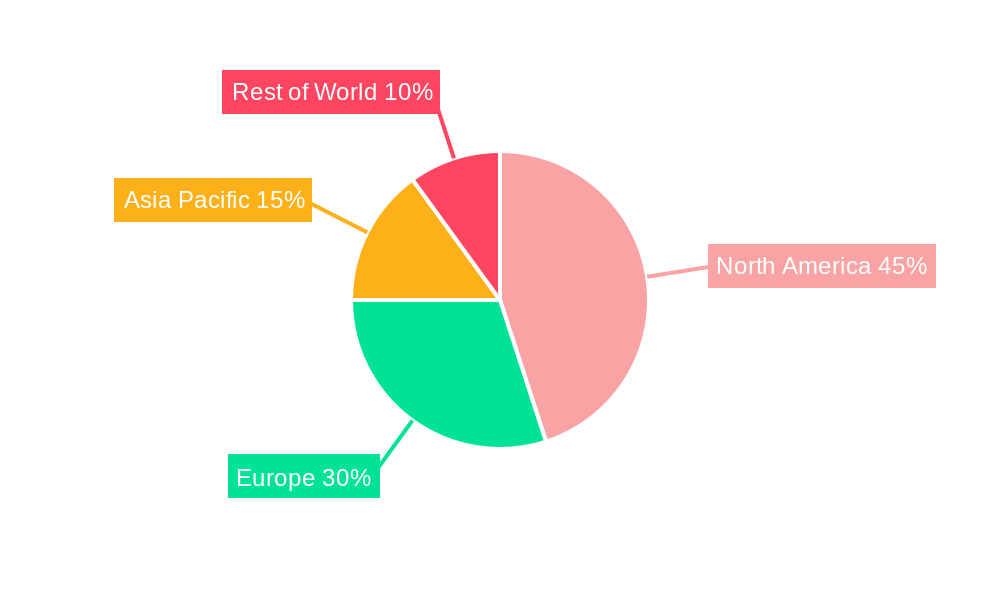
The Cell-bridging Bispecific Antibodies market is experiencing explosive growth, projected to reach $669.3 million in 2025 and exhibiting a remarkable Compound Annual Growth Rate (CAGR) of 39.0%. This rapid expansion is fueled by several key factors. Firstly, the increasing prevalence of hematological cancers and solid tumors, coupled with limitations of existing therapies, creates a significant unmet medical need. Secondly, the innovative mechanism of action of these antibodies, effectively bridging immune cells to cancer cells, offers superior efficacy and targeted treatment compared to traditional monoclonal antibodies. Thirdly, significant investments in R&D by major pharmaceutical companies like Amgen and Immunocore, along with emerging biotech firms, are driving the pipeline of novel cell-bridging bispecific antibodies, ensuring sustained market growth. The market segmentation reveals a strong focus on T-cell and NK-cell engagers, with applications spanning across hematological cancers and solid tumors. This diversity reflects the broad therapeutic potential of this technology. Regionally, North America currently dominates due to robust healthcare infrastructure, advanced research capabilities, and high adoption rates of novel therapies. However, other regions, especially Asia Pacific, are poised for substantial growth, driven by increasing healthcare expenditure and rising cancer incidence.
Continued expansion throughout the forecast period (2025-2033) is expected, driven by further clinical trial success, regulatory approvals, and expanding market awareness of cell-bridging bispecific antibodies' therapeutic advantages. While challenges remain, including high manufacturing costs and potential side effects requiring careful management, the significant therapeutic potential and ongoing technological advancements suggest this market will maintain its strong trajectory. The competitive landscape, characterized by both established pharmaceutical giants and innovative biotech companies, fosters innovation and contributes to the overall market dynamism. Further research and development will likely focus on improving efficacy, reducing toxicity, and expanding therapeutic applications, thereby sustaining the long-term growth potential of the Cell-bridging Bispecific Antibodies market.
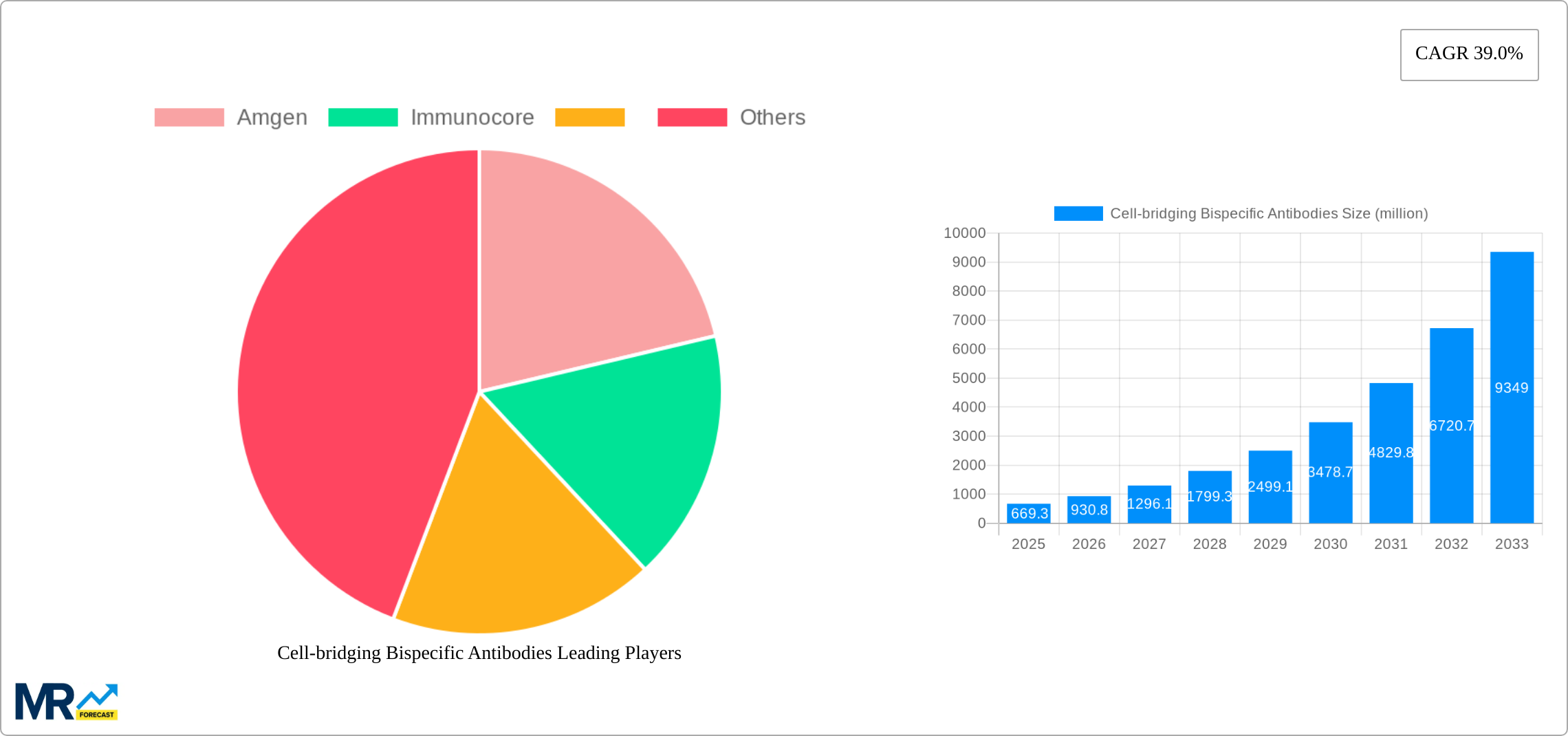
The cell-bridging bispecific antibody market is experiencing explosive growth, projected to reach multi-billion dollar valuations by 2033. Driven by a surge in approvals and pipeline candidates targeting hematological malignancies and solid tumors, this market segment is attracting significant investment from both established pharmaceutical giants and emerging biotech companies. The historical period (2019-2024) witnessed a gradual increase in market penetration, laying the groundwork for the remarkable expansion anticipated during the forecast period (2025-2033). Our analysis, based on data from 2019 to 2024 and projecting until 2033, with 2025 as both the base and estimated year, indicates a Compound Annual Growth Rate (CAGR) exceeding 20%. This growth is fueled by several factors, including improved efficacy compared to traditional monoclonal antibodies, the ability to target multiple cancer pathways simultaneously, and the increasing understanding of the complex interplay between immune cells and cancer cells. Key market insights reveal that T cell engagers currently hold the largest market share, though NK cell engagers are rapidly gaining ground, demonstrating strong potential for future market dominance. The success of these therapies hinges on overcoming challenges related to manufacturing complexity and managing potential adverse events, issues currently being addressed through technological innovation and refined clinical trial design. Amgen and Immunocore, among others, are key players shaping the landscape of this innovative therapeutic modality, investing heavily in research and development to expand their product portfolios and improve treatment outcomes for cancer patients. The market is segmented by type (T cell engagers, NK cell engagers), application (hematological cancers, solid tumors), and geography. The significant investments in R&D, coupled with the increasing number of clinical trials and regulatory approvals, point towards a continuously evolving and expanding market landscape.
The remarkable growth of the cell-bridging bispecific antibody market is driven by a confluence of factors. Firstly, the superior efficacy demonstrated by these therapies compared to traditional monoclonal antibodies is a major catalyst. Their ability to simultaneously engage two different targets, such as a tumor cell antigen and an immune effector cell (T cell or NK cell), allows for enhanced tumor cell killing and improved patient outcomes. This leads to higher remission rates and potentially longer survival times, making them highly attractive treatment options. Secondly, the growing understanding of the intricate mechanisms of cancer immunology has significantly contributed to the development of more effective and targeted cell-bridging bispecific antibodies. This deeper understanding allows for the design of molecules that precisely target specific immune pathways and tumor antigens, thus minimizing off-target effects and maximizing efficacy. Thirdly, the continuous technological advancements in antibody engineering are significantly improving the safety and production efficiency of these therapies. This results in cost-effectiveness and wider accessibility, pushing the market forward. Finally, increased funding for research and development, spurred by the promising clinical results, is further driving the innovation and expansion of this rapidly growing therapeutic area. The significant investments from both large pharmaceutical companies and smaller biotech firms demonstrate strong confidence in the future prospects of cell-bridging bispecific antibodies.
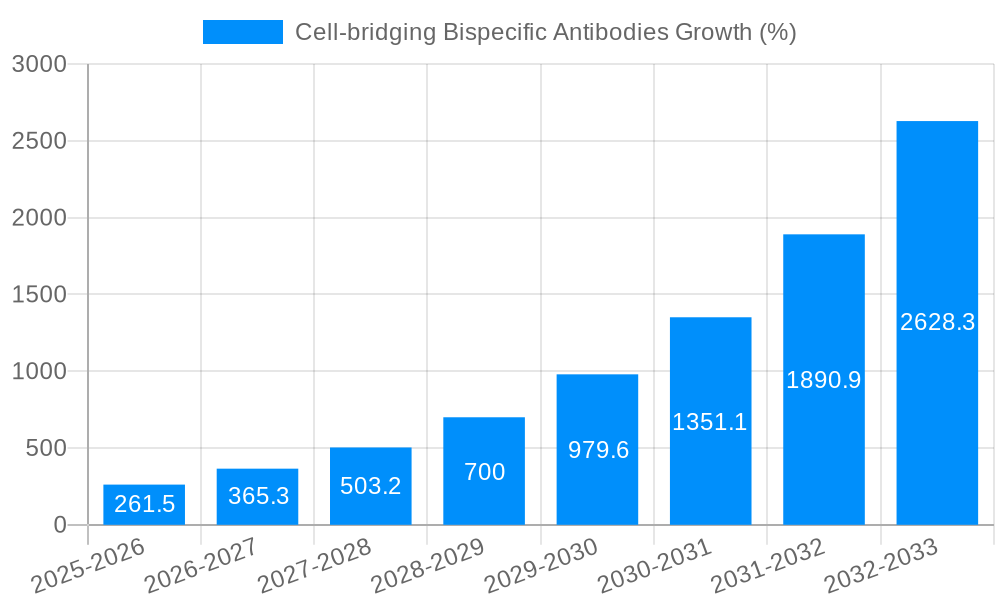
Despite the promising potential, several challenges and restraints hinder the widespread adoption of cell-bridging bispecific antibodies. Firstly, the manufacturing process is complex and expensive, limiting the accessibility and affordability of these therapies. The intricate engineering required to produce these highly specific molecules necessitates sophisticated manufacturing processes, leading to high production costs. Secondly, potential for adverse events, such as cytokine release syndrome (CRS) and neurotoxicity, represent a significant safety concern. While manageable in many cases, these side effects necessitate careful patient monitoring and effective management strategies, which increase the cost and complexity of treatment. Thirdly, the relatively short history of these therapies compared to traditional monoclonal antibodies results in limited long-term clinical data, making it challenging to fully assess their long-term efficacy and safety profiles. This limits the confidence of clinicians and regulatory bodies. Finally, the limited availability of effective biomarkers for predicting patient response further complicates treatment decisions and requires continued research and development to better stratify patients for treatment based on individual genetic profile and tumour features. Addressing these challenges requires collaborative efforts from researchers, manufacturers, and regulatory bodies to optimize manufacturing processes, refine treatment strategies, and expand the evidence base of these innovative therapies.
The North American market, particularly the United States, is expected to dominate the cell-bridging bispecific antibody market throughout the forecast period (2025-2033). This dominance is attributed to several factors, including:
High prevalence of cancer: The high incidence of hematological malignancies and solid tumors in the US fuels demand for effective cancer therapies.
Robust healthcare infrastructure: The well-developed healthcare infrastructure, including advanced research facilities and hospitals, supports clinical trials and the rapid adoption of new therapies.
High R&D investment: Significant government and private sector investments in pharmaceutical research and development drive innovation and the development of novel therapies.
Receptive regulatory environment: The Food and Drug Administration (FDA) has an efficient drug approval system and is proactive in facilitating the development and approval of novel cancer therapies.
Strong presence of major pharmaceutical companies: A large number of major biopharmaceutical companies headquartered in North America invest heavily in cell-bridging bispecific antibody research and development.
Segment Dominance: Within the segments, T cell engagers are expected to maintain a significant market share due to their proven efficacy in several hematological cancers. Their mechanism of action, directly activating cytotoxic T-cells to target and destroy cancer cells, makes them particularly effective in treating cancers that are sensitive to T-cell mediated immunity. However, the hematological cancers segment, driven by a growing understanding of the underlying immunology, and numerous therapies in development for a range of blood cancers, is projected to experience robust growth during the forecast period. This is further underpinned by the higher prevalence of hematological cancers compared to many solid tumor types, providing a larger target patient population. While solid tumors currently represent a smaller segment, ongoing research and development are focused on optimizing the design and efficacy of cell-bridging bispecific antibodies for various solid tumor types. This segment holds significant future potential as new, more effective agents enter the market.
The cell-bridging bispecific antibody market is experiencing significant growth due to the increasing prevalence of cancer globally, advancements in antibody engineering technology leading to improved efficacy and safety profiles, and the growing number of clinical trials and approvals of new molecules. Furthermore, substantial investments in R&D from major pharmaceutical companies are fueling innovation and expansion of this therapeutic area. The growing awareness and understanding among healthcare professionals and patients regarding the benefits of these targeted therapies will also drive the market's expansion.
This report provides a comprehensive analysis of the cell-bridging bispecific antibody market, covering market size, segmentation, trends, driving forces, challenges, key players, and significant developments. The data presented is based on rigorous market research and projections, allowing for informed decision-making by stakeholders in the pharmaceutical industry. The detailed market segmentation helps to identify key growth opportunities, while the analysis of key players offers valuable insights into competitive dynamics. This report is essential for anyone seeking to understand the current state and future trajectory of this rapidly growing market.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 39.0% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 39.0%.
Key companies in the market include Amgen, Immunocore, .
The market segments include Type, Application.
The market size is estimated to be USD 669.3 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Cell-bridging Bispecific Antibodies," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Cell-bridging Bispecific Antibodies, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.