1. What is the projected Compound Annual Growth Rate (CAGR) of the CD51 Antibody?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 CD51 Antibody
CD51 AntibodyCD51 Antibody by Type (Monoclonal, Polyclonal), by Application (Immunochemistry (IHC), Immunofluorescence (IF), Immunoprecipitation (IP), Western Blot (WB), ELISA, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The CD51 Antibody market is experiencing robust growth, driven by its critical role in immunochemistry, immunofluorescence, and other immunological assays essential for disease research and diagnostics. The market, valued at an estimated XXX million USD, is projected to expand at a Compound Annual Growth Rate (CAGR) of XX% from 2025 to 2033. This upward trajectory is primarily fueled by the increasing prevalence of chronic diseases, the growing demand for targeted therapies, and advancements in antibody production technologies. Key applications like Immunochemistry (IHC) and Western Blot (WB) are dominating market share due to their widespread use in academic research, pharmaceutical drug discovery, and clinical diagnostics. The increasing investment in life sciences research and development globally further bolsters the demand for high-quality CD51 antibodies.
The competitive landscape is characterized by the presence of numerous global and regional players, including Merck, Thermo Fisher Scientific, and GeneTex. These companies are actively engaged in product innovation, strategic collaborations, and geographic expansion to capture a larger market share. Emerging trends include the development of highly specific and sensitive monoclonal antibodies, as well as the growing adoption of multiplexing techniques in diagnostics, which will likely drive further market expansion. While the market is poised for significant growth, potential restraints such as the high cost of antibody production and stringent regulatory requirements could pose challenges. However, ongoing research and development efforts aimed at improving efficiency and reducing costs are expected to mitigate these challenges, ensuring sustained market dynamism.
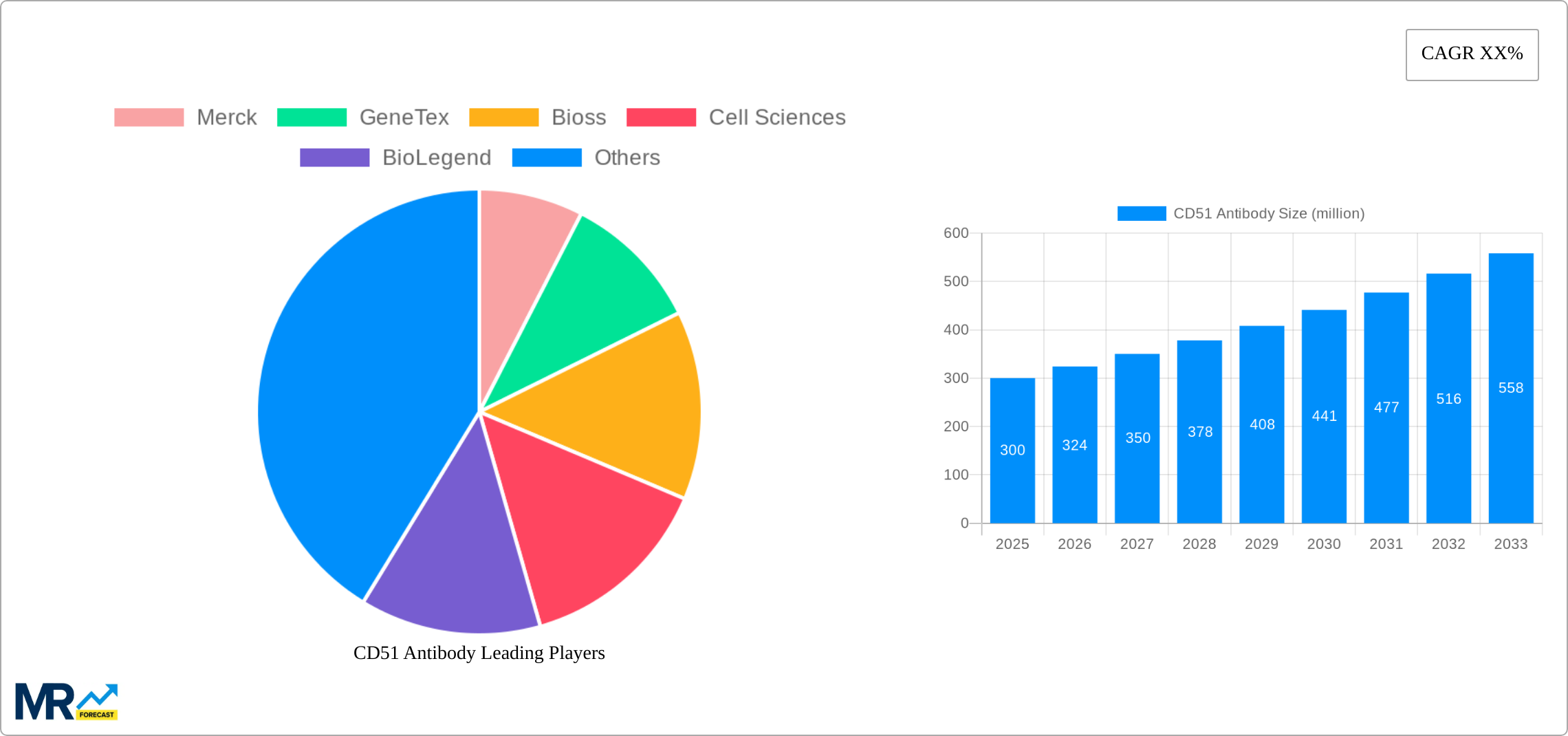
The CD51 antibody market is poised for significant expansion, driven by its pivotal role in understanding and targeting cellular processes implicated in a multitude of diseases, particularly in oncology and immunology. Our analysis indicates that the global CD51 antibody market, valued at approximately 50 million USD in the historical base year of 2019, has witnessed a steady upward trajectory, reaching an estimated 75 million USD by the base year of 2025. This growth is underpinned by an increasing number of research initiatives focusing on integrin alpha-V (ITGAV), the protein recognized by CD51 antibodies, which plays a crucial role in cell adhesion, migration, and proliferation. The forecast period, from 2025 to 2033, anticipates a compound annual growth rate (CAGR) of 6.5%, projecting the market to reach an impressive 120 million USD by 2033. Key market insights reveal a growing demand for highly specific and validated CD51 antibodies, particularly those suitable for multiplexing and advanced imaging techniques. The increasing sophistication of research methodologies, coupled with a deeper understanding of CD51's involvement in tumor microenvironment modulation and immune cell trafficking, is fueling this market expansion. Furthermore, the burgeoning biopharmaceutical industry's focus on developing novel therapeutic strategies targeting integrin pathways directly contributes to the sustained demand for high-quality CD51 antibodies for both research and pre-clinical development. The market is characterized by continuous innovation, with researchers actively exploring new applications of CD51 antibodies in diagnostics and therapeutic monitoring.
The CD51 antibody market is primarily propelled by the escalating global burden of diseases where CD51 plays a significant pathophysiological role. Oncology, in particular, stands out as a major driver, with CD51 being implicated in tumor angiogenesis, invasion, and metastasis. Researchers are increasingly utilizing CD51 antibodies to unravel the complex mechanisms of cancer progression and to identify potential therapeutic targets. This pursuit of novel anti-cancer therapies is creating substantial demand for reliable and sensitive CD51 antibodies for preclinical studies, drug screening, and biomarker discovery. Beyond oncology, the burgeoning field of immunology is also contributing to market growth. CD51 is expressed on various immune cells, including macrophages and T cells, influencing their activation, migration, and interaction with other cells. As research into immune-mediated diseases, autoimmune disorders, and inflammatory conditions intensifies, the need for CD51 antibodies to investigate these cellular interactions will undoubtedly surge. The continuous advancements in antibody engineering, leading to the development of monoclonal antibodies with enhanced specificity and affinity, are further bolstering market expansion. These sophisticated antibodies offer improved experimental outcomes, reducing non-specific binding and increasing the reliability of research findings, thereby driving their adoption across diverse research settings.
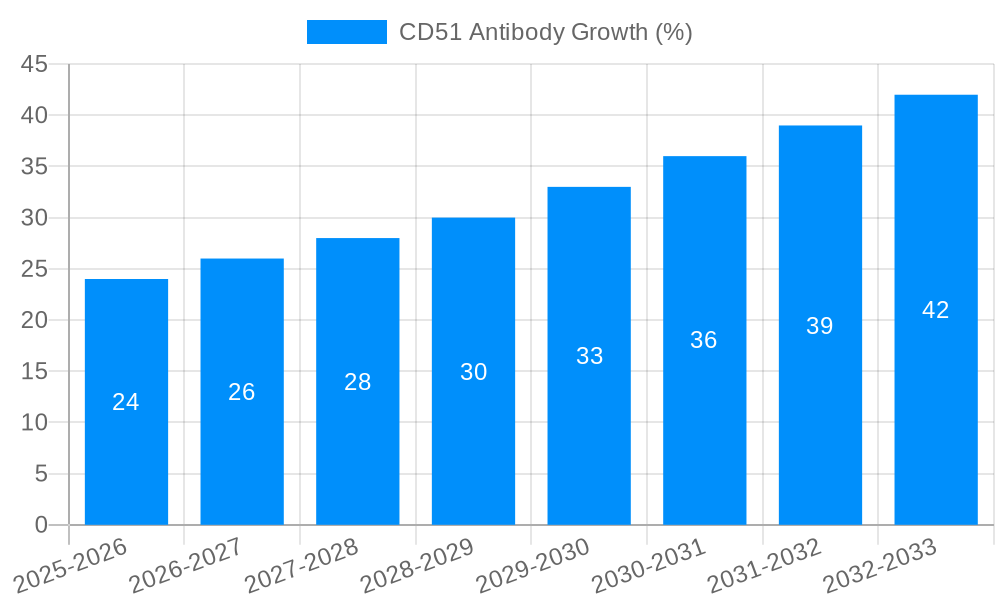
Despite the robust growth trajectory, the CD51 antibody market faces several challenges that could potentially restrain its full potential. One significant hurdle is the complexity and cost associated with antibody development and validation. Ensuring the specificity and sensitivity of CD51 antibodies, especially for novel research applications, requires extensive testing and optimization, which can be time-consuming and resource-intensive for manufacturers. The inherent variability in antibody performance across different experimental conditions and cell types can also lead to reproducibility issues in research, causing researchers to be cautious in their selections and demanding rigorous validation data from suppliers. Furthermore, the market is subject to stringent regulatory requirements for diagnostic and therapeutic applications, which can increase the time and cost for bringing new CD51 antibody-based products to market. The presence of a fragmented market with numerous players, while fostering competition, can also lead to price pressures and challenges in establishing consistent quality standards across all suppliers. Finally, the ongoing need for advanced instrumentation and technical expertise to effectively utilize CD51 antibodies in cutting-edge research, such as high-resolution imaging or advanced flow cytometry, can present a barrier to adoption for smaller research labs or those with limited budgets.
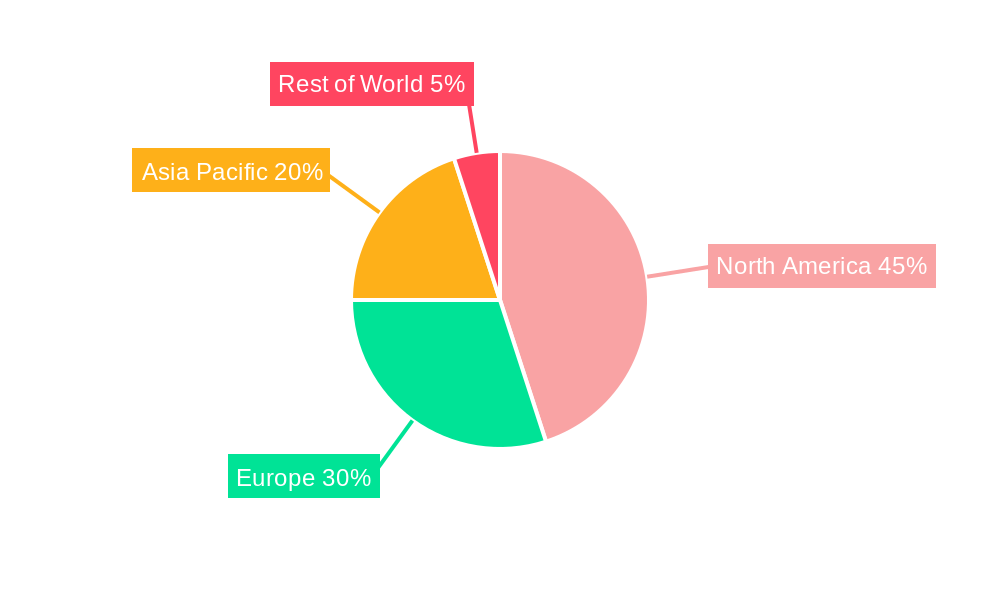
The North America region, specifically the United States, is projected to maintain its dominance in the global CD51 antibody market. This leadership is attributed to several intertwined factors.
Within the market segments, the Monoclonal type of CD51 antibodies is expected to dominate.
Among the applications, Immunochemistry (IHC) and Immunofluorescence (IF) are anticipated to be the leading segments driving demand.
The CD51 antibody industry's growth is significantly propelled by advancements in immunotherapy and precision medicine. As researchers delve deeper into the intricate roles of integrins in immune cell function and tumor evasion, CD51 antibodies are becoming indispensable tools for dissecting these complex pathways. The increasing focus on developing targeted therapies that modulate cell adhesion and migration for conditions like cancer and autoimmune diseases creates a robust demand. Furthermore, the expansion of high-throughput screening platforms and sophisticated imaging techniques demanding highly specific and validated reagents acts as a major growth catalyst, enabling more comprehensive and accurate research outcomes.
This report offers a comprehensive analysis of the CD51 antibody market, meticulously detailing trends, driving forces, and challenges from the historical period of 2019-2024, with an in-depth market estimation for the base year of 2025 and a detailed forecast extending to 2033. It provides crucial insights into the market's trajectory, identifying key growth catalysts, and highlighting the dominant regions and segments, particularly focusing on the dominance of North America and the monoclonal antibody segment, as well as the leading applications of IHC and IF. Furthermore, the report meticulously profiles the leading players in the market and chronicles significant developments that have shaped and will continue to shape the CD51 antibody sector. This comprehensive coverage ensures stakeholders have a clear and actionable understanding of the market dynamics.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Merck, GeneTex, Bioss, Cell Sciences, BioLegend, RayBiotech, Bio-Rad, Thermo Fisher Scientific, BD Biosciences, BosterBio, Enzo Life Sciences, LifeSpan BioSciences, Leinco Technologies, Antigenix America Inc., R&D Systems, Novus Biologicals, Miltenyi Biotec, SouthernBiotech, Biobyt, Jingjie PTM BioLab, .
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "CD51 Antibody," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the CD51 Antibody, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.