1. What is the projected Compound Annual Growth Rate (CAGR) of the Cartilage Regenerative Device?
The projected CAGR is approximately 17.14%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Cartilage Regenerative Device
Cartilage Regenerative DeviceCartilage Regenerative Device by Type (Biologic Soft Tissue Regeneration and Repair Devices, Biologic Cartilage Regeneration and Repair Devices, Biologic Tendon Regeneration and Repair Devices, Biologic Meniscus Regeneration and Repair Devices), by Application (Hospital, Clinic, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
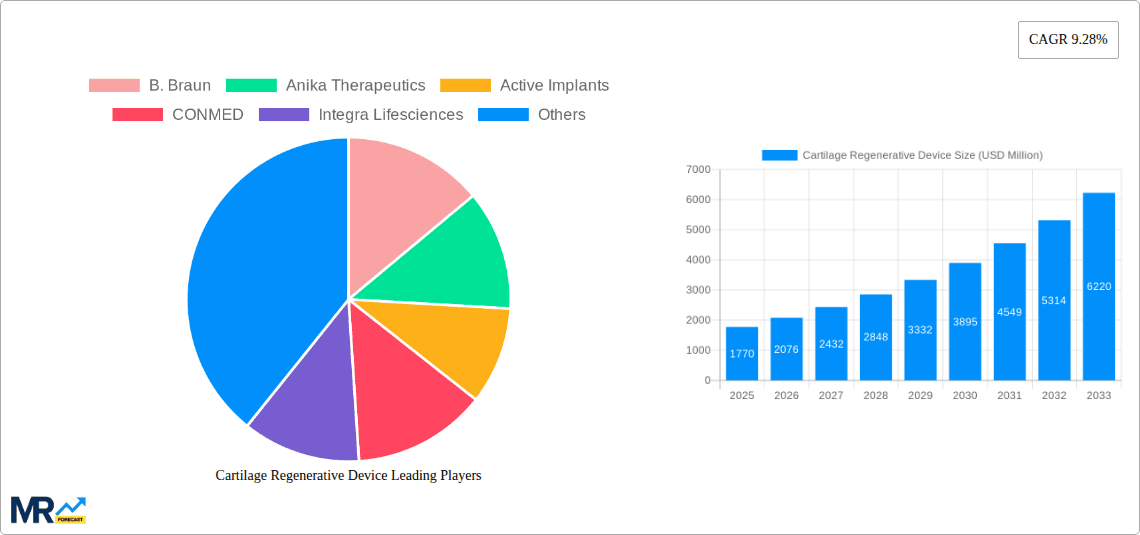
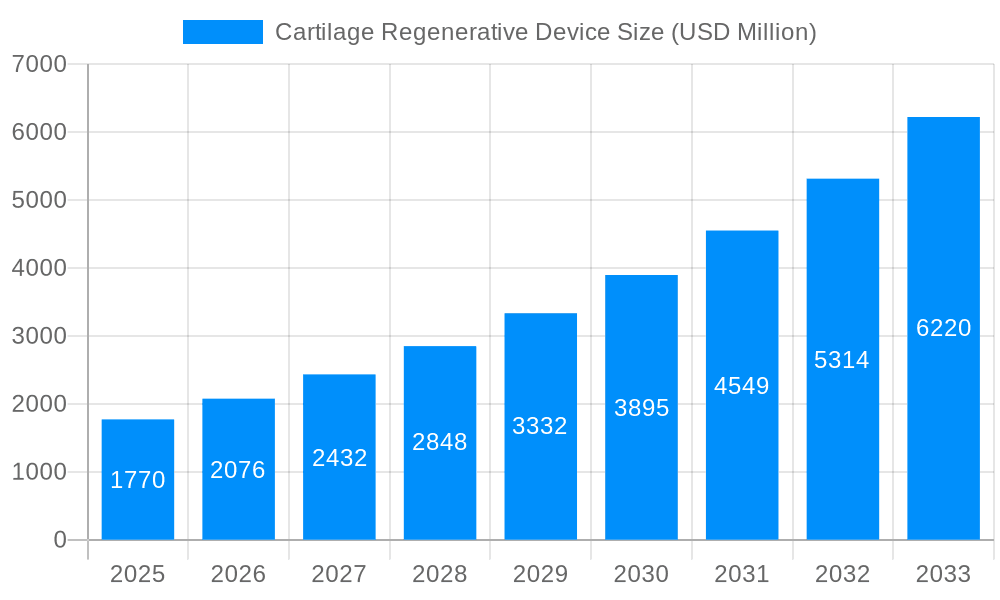
The Cartilage Regenerative Device market is poised for significant expansion, projected to reach an estimated USD 1.77 billion by 2025, with a robust Compound Annual Growth Rate (CAGR) of 17.14% through 2033. This remarkable growth is fueled by an increasing prevalence of sports-related injuries, the aging global population leading to degenerative joint conditions, and a growing demand for minimally invasive treatment options. Advances in biomaterial science and tissue engineering are continually introducing innovative devices that enhance cartilage repair and regeneration, offering patients alternatives to traditional surgical interventions and joint replacements. The market segments, including biologic soft tissue, cartilage, tendon, and meniscus regeneration and repair devices, all contribute to this upward trajectory, driven by a common need for effective solutions to musculoskeletal ailments. Hospitals and clinics are increasingly adopting these advanced regenerative technologies to improve patient outcomes and reduce recovery times, further accelerating market penetration.

The market is experiencing dynamic shifts driven by technological advancements and evolving healthcare paradigms. Key trends include the development of sophisticated biologic scaffolds, the integration of growth factors and stem cell technologies, and a greater focus on personalized regenerative medicine approaches. These innovations are directly addressing the limitations of existing treatments, offering the potential for complete tissue restoration rather than just symptom management. While the market presents immense opportunities, certain restraints, such as high treatment costs and the need for extensive clinical validation and regulatory approvals for novel technologies, need to be navigated. However, the persistent demand for improved quality of life, particularly among active individuals and the elderly, ensures a strong underlying market for cartilage regenerative devices. Major industry players like Johnson & Johnson, Stryker Corporation, Zimmer Biomet, and Smith & Nephew are actively investing in research and development, product launches, and strategic partnerships to capture a substantial share of this burgeoning market.

Here's a comprehensive report description for the Cartilage Regenerative Device market, incorporating your specific requirements:
The global Cartilage Regenerative Device market is poised for substantial expansion, projected to reach a significant valuation in the billions by 2033. This burgeoning market is characterized by a dynamic interplay of technological advancements, increasing demand for minimally invasive procedures, and a growing awareness of the benefits of regenerative therapies. XXX The study period from 2019 to 2033, with a base year of 2025, encompasses historical trends and forward-looking projections, highlighting a consistent upward trajectory. In the historical period of 2019-2024, the market witnessed steady growth driven by early adoption and initial research breakthroughs. The estimated year of 2025 marks a crucial juncture, where the market is expected to solidify its position and enter a phase of accelerated expansion. The forecast period of 2025-2033 anticipates this robust growth, fueled by further innovation and increasing clinical acceptance. Key insights reveal a strong preference for biologic solutions over traditional surgical interventions, driven by the potential for superior long-term outcomes and reduced recovery times. The market is also observing a growing interest in patient-specific treatments and the integration of advanced biomaterials. The increasing prevalence of osteoarthritis and sports-related injuries, particularly among aging populations and active individuals, is a primary driver for this market. Furthermore, the development of novel delivery systems and combination therapies is expected to unlock new therapeutic avenues and enhance treatment efficacy. The underlying trend points towards a paradigm shift in orthopedic care, moving away from purely palliative measures towards restorative and regenerative approaches that aim to restore native tissue function. This evolution is supported by significant investments in research and development by key players and a favorable regulatory environment for innovative medical devices. The market's trajectory is indicative of its crucial role in addressing unmet clinical needs and improving the quality of life for millions suffering from cartilage damage.
Several powerful forces are propelling the Cartilage Regenerative Device market forward. The escalating global burden of degenerative joint diseases, most notably osteoarthritis, coupled with the rising incidence of sports-related injuries, creates a substantial unmet clinical need for effective cartilage repair solutions. This demographic shift towards an aging population, who are more susceptible to these conditions, further amplifies the demand for treatments that can restore joint function and alleviate pain. Moreover, there is a discernible shift in patient and physician preference towards less invasive and more biologically driven therapeutic approaches. Regenerative devices offer the promise of stimulating the body's natural healing processes, potentially leading to better long-term outcomes, reduced morbidity, and quicker rehabilitation compared to traditional surgical interventions like joint replacements. Advances in biomaterials science, cell biology, and tissue engineering are continuously paving the way for more sophisticated and effective regenerative devices, offering enhanced efficacy and safety profiles. These technological advancements are supported by increasing investments in research and development by both established medical device manufacturers and innovative biotech startups, fostering a vibrant ecosystem of innovation.
Despite the promising growth trajectory, the Cartilage Regenerative Device market faces several significant challenges and restraints that could temper its expansion. One of the primary hurdles is the high cost of developing and implementing these advanced regenerative therapies. Research, clinical trials, and manufacturing of sophisticated biologic devices are inherently expensive, which can translate into high treatment costs for patients and healthcare systems. This cost factor can limit widespread adoption, particularly in regions with constrained healthcare budgets. Regulatory hurdles and the time-consuming approval processes for novel regenerative devices also pose a significant challenge. Demonstrating the safety and efficacy of these complex biological products requires rigorous scientific evidence and extensive clinical validation, which can be a lengthy and resource-intensive undertaking. Limited reimbursement policies in certain healthcare systems can further impede market penetration. If regenerative treatments are not adequately covered by insurance, patients may be reluctant to opt for them, even if they offer superior long-term benefits. Furthermore, a lack of standardized treatment protocols and long-term clinical data can create uncertainty among healthcare providers regarding the efficacy and predictability of outcomes. Building physician confidence and educating the medical community about the benefits and appropriate use of these devices is crucial but requires sustained effort. Lastly, challenges related to the scalability of manufacturing and ensuring consistent product quality for complex biologic agents can impact market supply and demand dynamics.
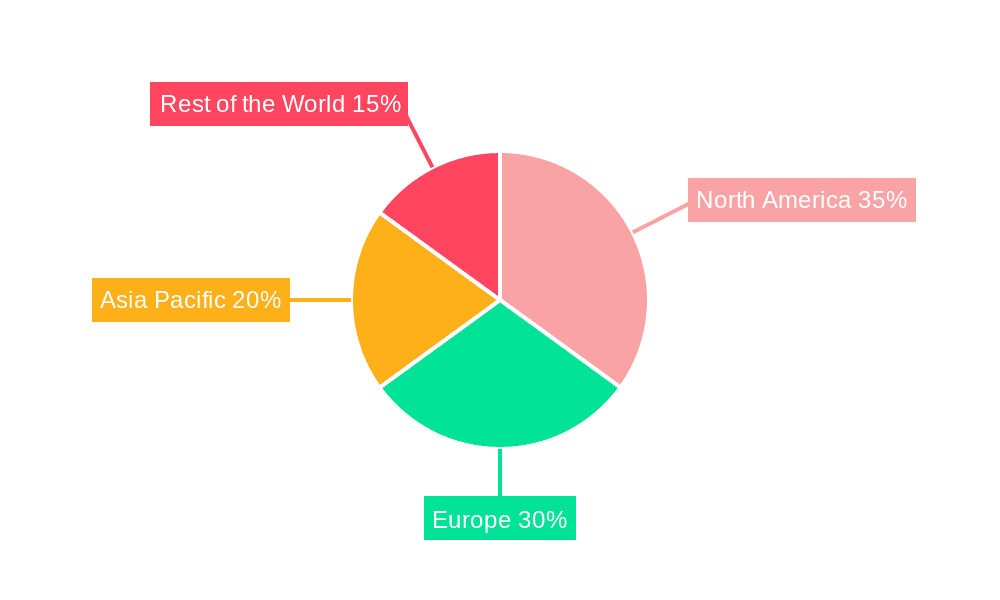
The Cartilage Regenerative Device market is anticipated to witness significant dominance from specific regions and segments, driven by a confluence of factors including healthcare infrastructure, research capabilities, and patient demographics.
North America, particularly the United States, is poised to emerge as a leading region, driven by:
Europe is another significant market, with countries like Germany, the UK, and France showing strong potential, owing to:
In terms of segment dominance, the Biologic Cartilage Regeneration and Repair Devices segment is expected to be the frontrunner. This dominance is driven by:
While other segments like Biologic Soft Tissue Regeneration and Repair Devices are also significant, the specific focus and growing efficacy of dedicated cartilage regeneration solutions position it for leading market share. Within the Application segment, Hospitals are expected to be the primary point of care for these advanced treatments, given their established infrastructure for complex surgical procedures, access to specialized medical professionals, and capabilities for managing post-operative care.
The Cartilage Regenerative Device industry's growth is significantly catalyzed by several key factors. The relentless pursuit of improved patient outcomes and the desire to move beyond palliative care are driving innovation. Advances in biomaterials science are enabling the development of scaffolds that better mimic the native extracellular matrix, promoting cell adhesion, proliferation, and differentiation. Furthermore, breakthroughs in cell therapy, including the use of stem cells and engineered cells, offer the potential for more potent and targeted regenerative responses. Increased awareness and acceptance of regenerative medicine among both clinicians and patients, coupled with supportive regulatory pathways for innovative therapies, are also acting as powerful growth catalysts, paving the way for wider adoption.
This report offers an in-depth and comprehensive analysis of the Cartilage Regenerative Device market, providing critical insights for stakeholders. XXX The study meticulously examines market trends, growth drivers, and potential challenges, offering a holistic view from the historical period of 2019-2024 to the projected landscape up to 2033. It delves into the specific dynamics of various segments, including Biologic Soft Tissue Regeneration and Repair Devices, Biologic Cartilage Regeneration and Repair Devices, Biologic Tendon Regeneration and Repair Devices, and Biologic Meniscus Regeneration and Repair Devices, alongside application-specific insights from Hospitals, Clinics, and Others. The report highlights key regional markets, with a particular focus on North America and Europe, and identifies dominant segments crucial for strategic decision-making. Furthermore, it provides a detailed overview of the leading players and significant developments shaping the industry, enabling stakeholders to identify opportunities, mitigate risks, and formulate robust strategies for success in this rapidly evolving and high-growth sector.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 17.14% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 17.14%.
Key companies in the market include B. Braun, Anika Therapeutics, Active Implants, CONMED, Integra Lifesciences, Arthrex, Aesculap Biologics, Johnson & Johnson, Organogenesis Holdings, Stryker Corporation, Zimmer Biomet, Smith & Nephew, RTI Surgical, .
The market segments include Type, Application.
The market size is estimated to be USD XXX N/A as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in N/A.
Yes, the market keyword associated with the report is "Cartilage Regenerative Device," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Cartilage Regenerative Device, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.