1. What is the projected Compound Annual Growth Rate (CAGR) of the Pharmacovigilance Software?
The projected CAGR is approximately 5.6%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Pharmacovigilance Software
Pharmacovigilance SoftwarePharmacovigilance Software by Type (ADR Reporting, Drug Safety Audits, Issue Tracking, Fully Integrated Software), by Application (Pharma & biotech companies, CROs, BPOs, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
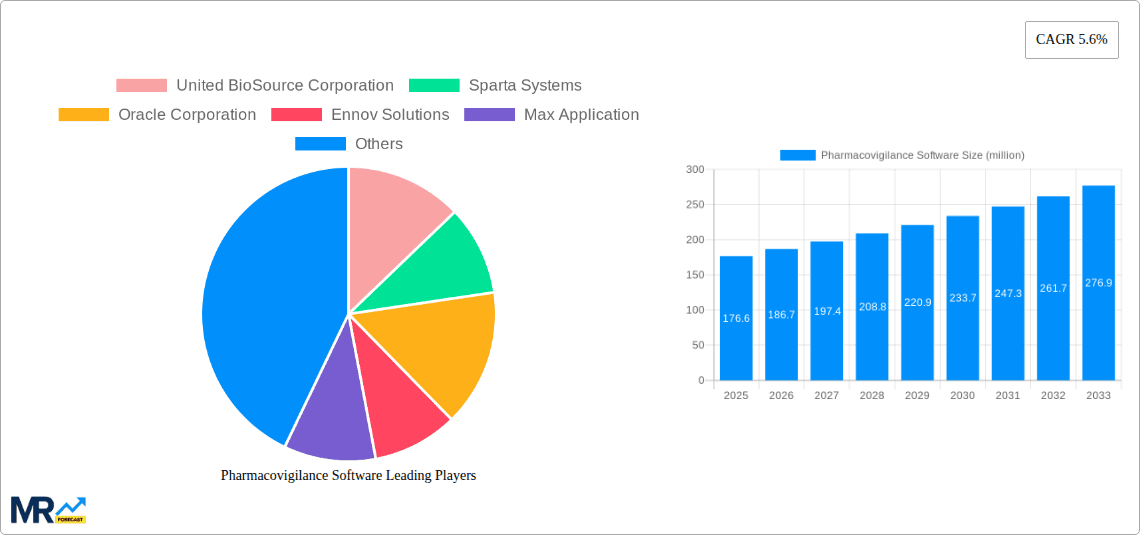
The global pharmacovigilance software market, valued at $176.6 million in 2025, is poised for robust growth, exhibiting a compound annual growth rate (CAGR) of 5.6% from 2025 to 2033. This expansion is fueled by several key drivers. Increasing regulatory scrutiny and the need for enhanced drug safety monitoring are paramount, pushing pharmaceutical and biotechnology companies, Contract Research Organizations (CROs), and Business Process Outsourcing (BPO) providers to adopt sophisticated software solutions. The rising volume of adverse drug reaction (ADR) reports necessitates efficient and automated systems for data management, analysis, and reporting. Furthermore, the growing adoption of cloud-based solutions and the integration of artificial intelligence (AI) and machine learning (ML) for advanced signal detection and risk assessment are driving market expansion. The market is segmented by software type (ADR reporting, drug safety audits, issue tracking, fully integrated software) and application (pharma & biotech companies, CROs, BPOs, others). North America currently holds a significant market share due to stringent regulations and the presence of major pharmaceutical players, followed by Europe and Asia Pacific.

The market's future trajectory is shaped by several trends. The increasing adoption of integrated platforms offering a comprehensive suite of pharmacovigilance functions is a notable trend. This consolidation simplifies workflows and improves data consistency. The development of advanced analytics capabilities within pharmacovigilance software enables quicker identification of safety signals and facilitates proactive risk management. However, challenges remain. High initial investment costs and the complexities associated with data integration and validation across diverse systems can act as restraints for smaller companies. The need for skilled professionals to effectively manage and interpret data generated by these sophisticated systems also poses a significant hurdle. Despite these challenges, the long-term prospects for the pharmacovigilance software market remain highly positive, fueled by increasing regulatory pressures, technological advancements, and the rising need for robust drug safety monitoring globally.
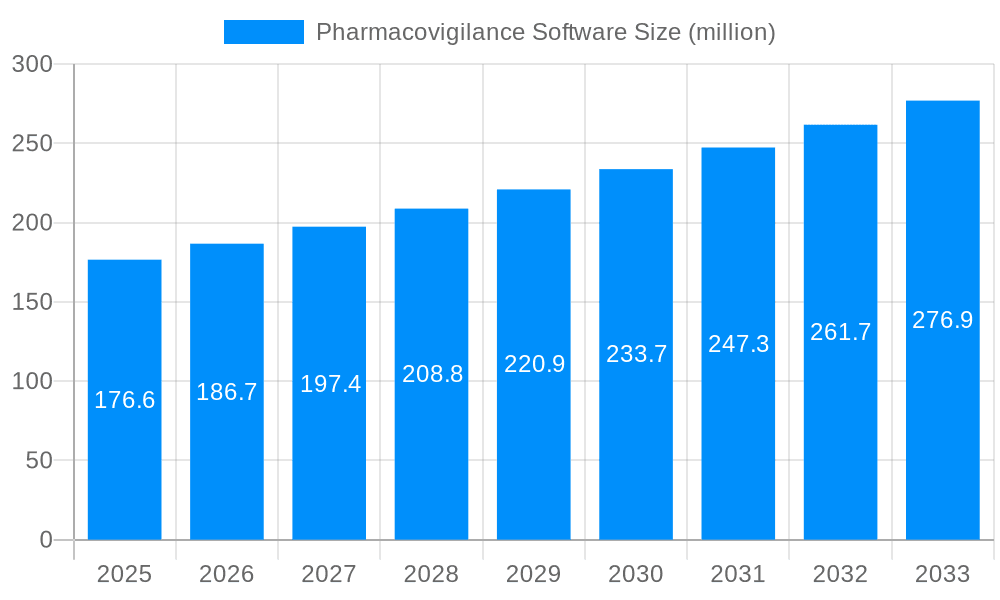

The global pharmacovigilance software market is experiencing robust growth, projected to reach multi-billion dollar valuations by 2033. Driven by increasing regulatory scrutiny, a surge in drug approvals, and the expanding adoption of digital health technologies, the market demonstrates a consistent upward trajectory. The historical period (2019-2024) witnessed significant market expansion, laying the groundwork for substantial growth during the forecast period (2025-2033). Key market insights reveal a strong preference for fully integrated software solutions, offering streamlined workflows and enhanced data management capabilities. The rising adoption of cloud-based solutions is further fueling market expansion, enabling scalability, accessibility, and cost-effectiveness. Pharma & biotech companies represent the largest segment of the market, followed by CROs (Contract Research Organizations) and BPOs (Business Process Outsourcing) organizations. However, the "Others" segment is expected to exhibit significant growth owing to increasing adoption by smaller pharmaceutical entities and related healthcare providers. The market is characterized by intense competition among established players and emerging technology providers, leading to continuous innovation and the development of sophisticated features like advanced analytics and AI-powered safety signal detection. The estimated market value in 2025 exceeds several hundred million dollars, reflecting the considerable investment and growth potential within this critical area of pharmaceutical development and post-market surveillance. Furthermore, the increasing complexity of drug development pipelines and the necessity for robust safety monitoring are primary catalysts in shaping market trends. This necessitates the adoption of advanced solutions that offer enhanced efficiency, data integrity, and regulatory compliance.
Several factors are driving the expansion of the pharmacovigilance software market. Stringent regulatory requirements mandating robust safety reporting and surveillance systems are paramount. Global regulatory bodies such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency) are increasingly demanding sophisticated pharmacovigilance systems, pushing companies to adopt advanced software solutions. The growing volume of adverse drug reaction (ADR) reports necessitates automated and efficient systems to manage and analyze this vast amount of data, which traditional methods are ill-equipped to handle. The need for real-time safety signal detection is another crucial factor, enabling proactive intervention and risk mitigation. The integration of artificial intelligence (AI) and machine learning (ML) into pharmacovigilance software is significantly improving the speed and accuracy of safety signal detection, further driving market growth. Moreover, the rising adoption of cloud-based solutions offers scalability, accessibility, and cost-effectiveness, appealing to organizations of varying sizes and budgets. The increasing global adoption of electronic health records (EHRs) also creates opportunities for seamless integration with pharmacovigilance systems, promoting data interoperability and enhanced safety monitoring.
Despite the significant growth potential, the pharmacovigilance software market faces several challenges. High initial investment costs associated with implementing and maintaining sophisticated software systems can be a barrier for smaller companies. Data security and privacy concerns are paramount, requiring robust security protocols and compliance with regulations like GDPR (General Data Protection Regulation). The complexity of integrating different systems and data sources presents a significant hurdle, demanding seamless interoperability between various platforms. The need for highly skilled professionals to operate and manage these complex systems leads to talent shortages and increased labor costs. Furthermore, the continuous evolution of regulatory requirements necessitates ongoing software updates and maintenance, potentially incurring substantial costs. The need for user-friendly interfaces and training programs to ensure effective adoption across the organization is also a key consideration. Finally, the constant need for adaptation to emerging technologies and evolving regulatory frameworks poses ongoing challenges for both software providers and end-users.
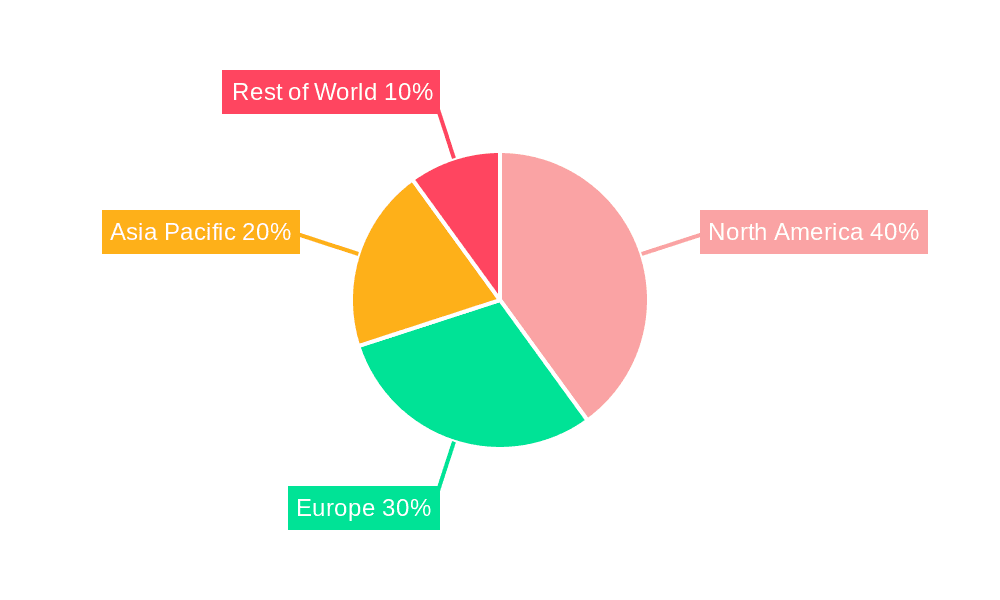
The North American market, particularly the United States, is expected to hold a significant share of the global pharmacovigilance software market throughout the forecast period. This is primarily attributed to the presence of major pharmaceutical companies, stringent regulatory frameworks, and early adoption of advanced technologies. Similarly, the European Union shows substantial growth potential, driven by strong regulatory compliance needs and the increasing focus on patient safety. The Asia-Pacific region is also witnessing significant growth due to expanding pharmaceutical markets and rising investment in healthcare infrastructure in countries like India and China.
Dominant Segment: The Fully Integrated Software segment is poised for significant growth. This is because fully integrated systems offer end-to-end solutions, streamlining workflows and improving data management, thereby enhancing efficiency and compliance. These systems seamlessly integrate various functions like ADR reporting, drug safety audits, issue tracking, and data analytics, providing a holistic view of safety data. This allows for a more effective and proactive approach to pharmacovigilance. In contrast to disparate systems, fully integrated solutions eliminate data silos and manual data transfer, reducing the risk of errors and improving overall data quality. The increased efficiency and reduced operational costs associated with these solutions make them attractive to companies of all sizes.
Other Significant Segments: While fully integrated software is dominating, the ADR Reporting segment also maintains strong growth potential due to the ever-increasing volume of adverse drug reactions requiring efficient reporting and analysis. The Pharma & biotech companies application segment remains the largest user base due to their direct involvement in drug development and post-market surveillance. However, the CROs and BPOs segments are experiencing fast growth as they increasingly adopt these systems to manage pharmacovigilance activities for their clients.
The pharmacovigilance software industry’s growth is catalyzed by several factors: rising regulatory pressures necessitating comprehensive safety monitoring; increasing complexity of drug development; escalating demand for real-time safety signal detection; the integration of AI and ML for enhanced data analysis; and the growing adoption of cloud-based solutions for improved scalability and cost-effectiveness. These factors collectively drive the demand for sophisticated and efficient pharmacovigilance software, propelling significant market expansion.
This report provides a comprehensive overview of the pharmacovigilance software market, offering detailed insights into market trends, driving forces, challenges, key players, and significant developments. The report utilizes extensive market research and data analysis to provide accurate forecasts and projections for the future. It caters to a wide range of stakeholders, including pharmaceutical companies, CROs, BPOs, regulatory bodies, investors, and technology providers, offering valuable information to make informed business decisions within this rapidly evolving sector. The detailed segmentation and regional analysis facilitates a thorough understanding of the market dynamics and growth potential.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.6% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 5.6%.
Key companies in the market include United BioSource Corporation, Sparta Systems, Oracle Corporation, Ennov Solutions, Max Application, EXTEDO GmbH, AB Cube, Relsys, Online Business Applications, ArisGlobal, .
The market segments include Type, Application.
The market size is estimated to be USD 176.6 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Pharmacovigilance Software," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Pharmacovigilance Software, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.