1. What is the projected Compound Annual Growth Rate (CAGR) of the Newborn Genetic Screening?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Newborn Genetic Screening
Newborn Genetic ScreeningNewborn Genetic Screening by Type (Dry blood spot tests, Hearing screening tests, CCHD screening tests), by Application (Clinical Laboratories, Hospitals), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The Newborn Genetic Screening market is experiencing robust growth, driven by increasing awareness of genetic disorders, advancements in screening technologies, and expanding newborn screening panels. The market's size in 2025 is estimated at $2.5 billion, reflecting a compound annual growth rate (CAGR) of 8% from 2019 to 2024. This growth is projected to continue through 2033, propelled by factors such as the rising prevalence of genetic diseases, increased adoption of next-generation sequencing (NGS) technologies offering faster and more comprehensive screening, and expanding government initiatives promoting early diagnosis and intervention. Furthermore, the decreasing cost of genetic testing is making these crucial screenings more accessible to a wider population, further fueling market expansion.
However, challenges remain. The market faces restraints like the high cost of advanced testing technologies, ethical concerns surrounding genetic information privacy and informed consent, and varying regulatory frameworks across different regions. Despite these, the significant benefits of early diagnosis and intervention for newborns with genetic conditions are undeniably driving demand. Market segmentation is primarily driven by technology type (e.g., tandem mass spectrometry, microarray, NGS), test type (e.g., carrier screening, newborn screening panels), and end-user (hospitals, laboratories, clinics). Key players such as Trivitron Healthcare, Masimo Corporation, and others are continuously innovating and expanding their product portfolios to meet the growing needs of this rapidly evolving market. The increasing focus on personalized medicine further strengthens the long-term prospects for the newborn genetic screening market.
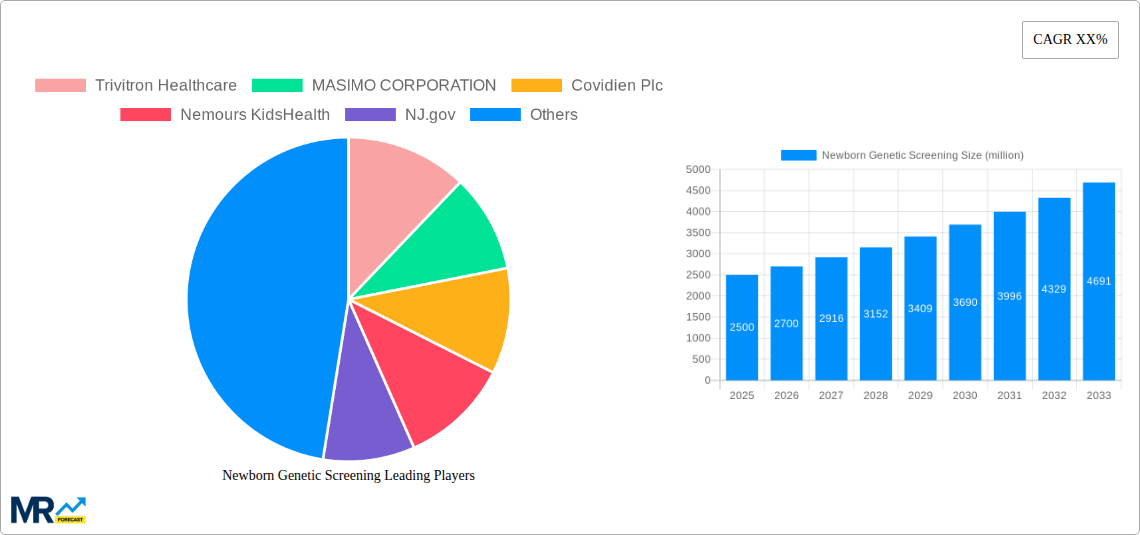
The global newborn genetic screening market is experiencing robust growth, projected to reach multi-million dollar valuations by 2033. Driven by advancements in technology, increased awareness of genetic disorders, and expanding government support for early intervention programs, the market demonstrates significant potential. The historical period (2019-2024) saw steady expansion, with the base year of 2025 marking a crucial point of inflection. Our estimations for 2025 indicate a substantial market size, setting the stage for impressive growth during the forecast period (2025-2033). This growth is fueled not only by technological innovation like next-generation sequencing (NGS) and tandem mass spectrometry (TMS), but also by a shift in healthcare priorities towards preventative and personalized medicine. The increasing affordability and accessibility of these technologies are further driving adoption. Furthermore, the rising prevalence of treatable genetic disorders, coupled with improved understanding of their long-term implications, is compelling healthcare providers and parents to prioritize early screening. The market is seeing a gradual shift towards expanded carrier screening panels, offering comprehensive evaluations beyond the traditionally screened conditions. This broadened scope contributes to the overall market expansion, attracting significant investments from both private and public sectors. The integration of data analytics and artificial intelligence is also becoming increasingly prominent, enabling more accurate diagnoses, personalized treatment plans, and improved risk assessment. This comprehensive approach to newborn genetic screening is reshaping the landscape of neonatal care, paving the way for healthier outcomes and improved quality of life for affected individuals.
Several key factors contribute to the rapid expansion of the newborn genetic screening market. Technological advancements, particularly in NGS and TMS, have significantly reduced the cost and time required for testing, making it more accessible and affordable. This increased accessibility is further amplified by government initiatives promoting early detection and intervention programs for genetic disorders. Growing public awareness and understanding of the benefits of early diagnosis are crucial, empowering parents to make informed decisions about their child's health. The ability to identify and treat conditions early minimizes long-term health complications and improves overall quality of life. Moreover, the growing prevalence of treatable genetic disorders necessitates expanded screening capabilities, driving demand for more comprehensive panels. The increasing focus on personalized medicine and preventative healthcare is aligning perfectly with the objectives of newborn genetic screening, fostering wider acceptance and adoption across healthcare systems. Furthermore, the proactive approach of identifying potential risks and tailoring medical interventions contributes to cost-effectiveness in the long run by preventing expensive treatments later in life.
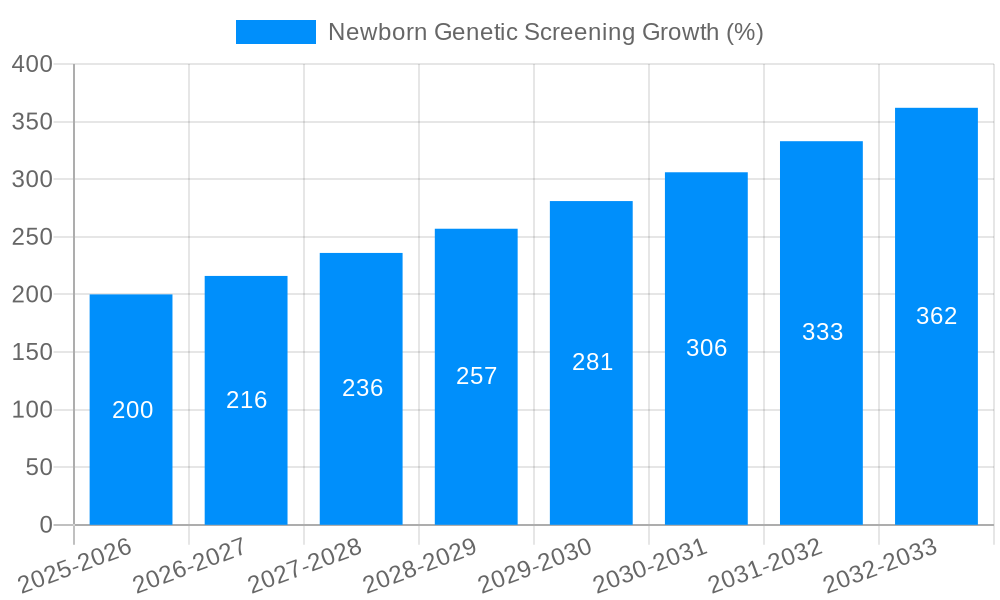
Despite the significant growth potential, the newborn genetic screening market faces certain challenges. One major hurdle is the ethical considerations surrounding genetic testing, including issues related to privacy, informed consent, and the potential for genetic discrimination. Establishing clear guidelines and regulations is crucial for addressing these concerns and building public trust. The high cost of some advanced technologies, particularly NGS, remains a significant barrier, limiting access for some populations. This necessitates ongoing research and development to find cost-effective solutions while maintaining high levels of accuracy and reliability. Another challenge involves interpreting the results accurately, especially in complex cases with multiple variants of unknown significance. The need for skilled professionals and robust data interpretation systems is vital to ensure accurate diagnoses and prevent misinterpretations. Furthermore, the availability of trained personnel to handle the increasing volume of tests and manage patient follow-up poses a significant challenge to the expansion of the market. Finally, ensuring equitable access to newborn genetic screening across diverse geographical locations and socioeconomic populations is paramount to realizing the full potential of this life-saving technology.
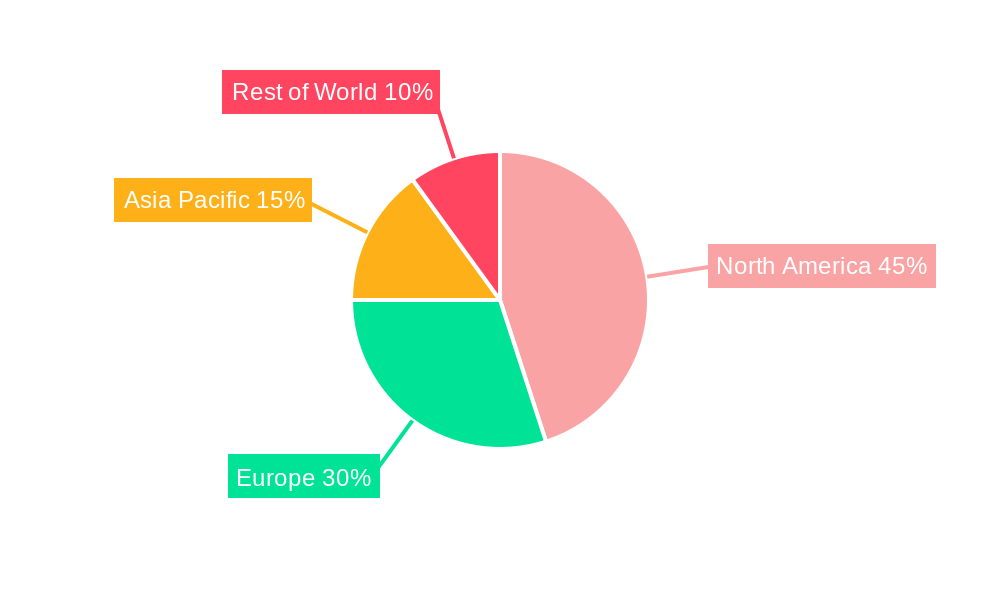
The North American market is expected to dominate the newborn genetic screening market throughout the forecast period. This is due to a combination of factors, including advanced healthcare infrastructure, high adoption rates of advanced technologies, and significant investments in research and development.
Europe is another significant market, although slightly behind North America in terms of adoption. The market in Asia-Pacific is expected to grow rapidly during the forecast period, fueled by increasing awareness, growing healthcare infrastructure, and increasing affordability of technologies.
The newborn genetic screening market is being propelled by advancements in technology resulting in faster, more affordable, and more accurate testing. Increased public awareness of genetic diseases and the benefits of early detection are also major drivers. Government initiatives supporting early intervention programs and improved reimbursement policies are further stimulating market growth.
This report provides a comprehensive analysis of the newborn genetic screening market, including detailed market sizing, segmentation, trends, and future projections. It also offers insights into the key players, technologies, and regulatory landscape. The report helps stakeholders to understand the opportunities and challenges within the market and make informed decisions for future growth.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Trivitron Healthcare, MASIMO CORPORATION, Covidien Plc, Nemours KidsHealth, NJ.gov, Agilent Technologies, Ge Healthcare, PERKINELMER, Natus Medical Incorporated, Waters, Bio-Rad Laboratories, Ab Sciex LLC.
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Newborn Genetic Screening," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Newborn Genetic Screening, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.