1. What is the projected Compound Annual Growth Rate (CAGR) of the Clinical Trial Software?
The projected CAGR is approximately 13.7%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Clinical Trial Software
Clinical Trial SoftwareClinical Trial Software by Type (Web-based CTMS, On-premise, Cloud-based CTMS), by Application (Pharmaceutical and Biopharmaceutical Companies, Clinical Research Organizations, Healthcare Providers), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The global Clinical Trial Management System (CTMS) software market is poised for significant expansion, driven by escalating trial complexity, the imperative for superior data management and collaboration, and the widespread adoption of cloud-based technologies. The market, projected to reach $1017 million by 2025, is anticipated to grow at a Compound Annual Growth Rate (CAGR) of 13.7% from 2025 to 2033. Key growth drivers include the burgeoning pharmaceutical and biopharmaceutical sectors initiating more intricate trials, the increasing demand for efficient data handling and regulatory adherence, and the growing preference for scalable, accessible, and cost-effective cloud CTMS solutions over on-premise alternatives. North America currently leads the market, supported by robust research infrastructure and a high density of pharmaceutical enterprises. However, the Asia-Pacific region is expected to exhibit substantial growth, fueled by escalating healthcare investments and a rising volume of clinical trials. The market segmentation highlights a distinct preference for cloud-based CTMS due to its advantages in accessibility, cost optimization, and streamlined implementation. Pharmaceutical and biopharmaceutical companies represent the primary adopters, followed by Contract Research Organizations (CROs).
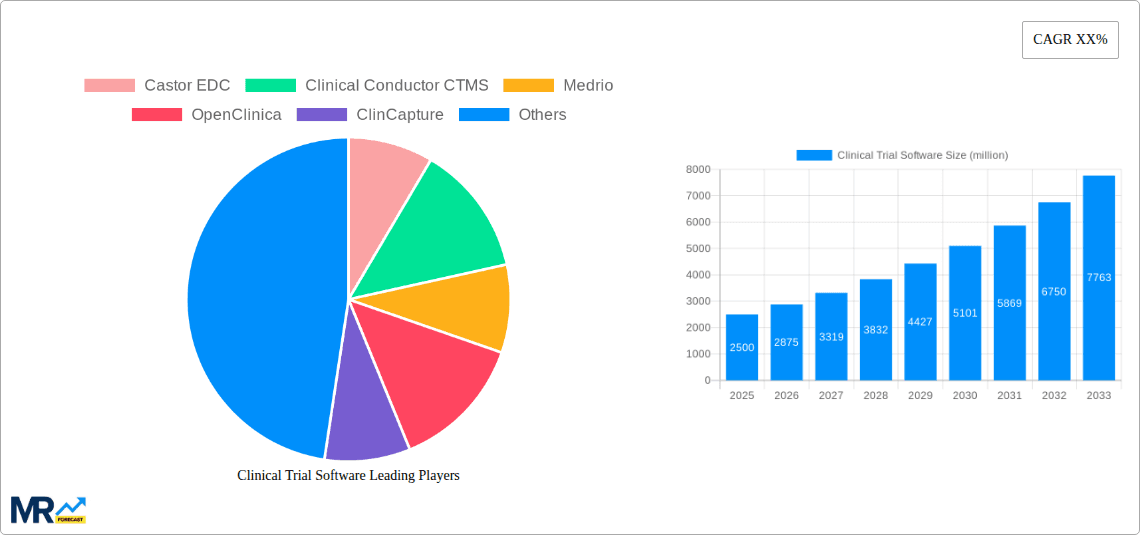

The competitive environment features established vendors offering comprehensive CTMS solutions alongside specialized providers focusing on specific trial management functionalities. Leading companies are continuously innovating, integrating AI-driven data analytics, enhancing interoperability with other clinical trial tools, and strengthening data security protocols. Despite robust growth, the market encounters challenges such as considerable initial investment in software and training, data security and privacy concerns, and the intricate regulatory landscape governing clinical trial data. Nevertheless, the CTMS market outlook remains exceptionally positive, forecasting sustained growth and increasing global penetration as the demand for efficient and robust clinical trial management intensifies.
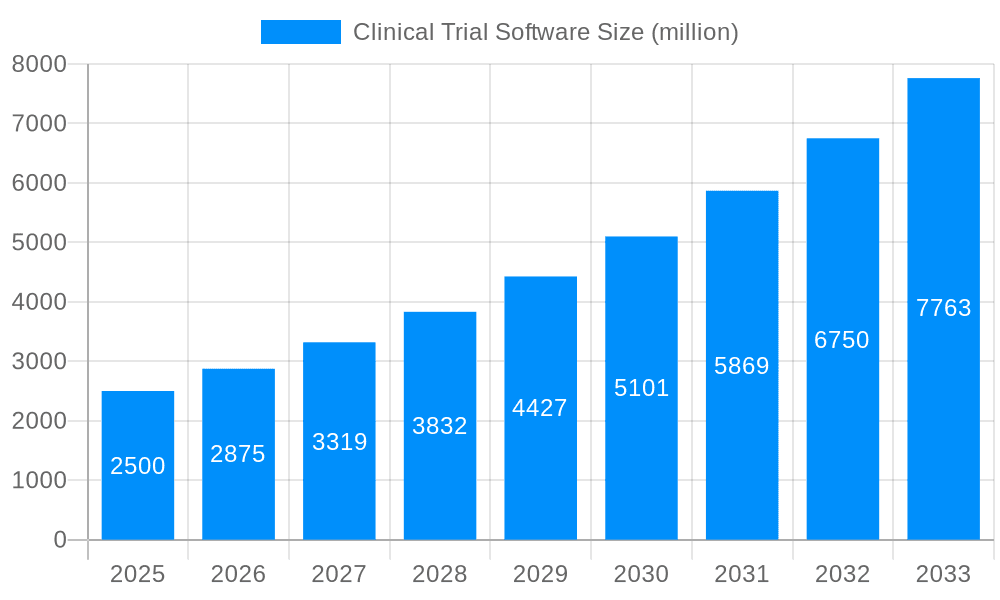

The global clinical trial software market is experiencing robust growth, projected to reach multi-billion dollar valuations by 2033. Driven by increasing clinical trial complexity, stringent regulatory requirements, and the need for efficient data management, the market exhibits a clear shift towards cloud-based solutions. The historical period (2019-2024) saw significant adoption of web-based Clinical Trial Management Systems (CTMS) and Electronic Data Capture (EDC) systems, particularly among pharmaceutical and biopharmaceutical companies. This trend is expected to accelerate during the forecast period (2025-2033), fueled by the scalability, accessibility, and cost-effectiveness of cloud-based infrastructure. The estimated market value in 2025 is in the hundreds of millions, reflecting the substantial investment in digital transformation within the clinical research sector. Moreover, the rising adoption of Artificial Intelligence (AI) and machine learning (ML) for data analysis and predictive modeling is further enhancing the capabilities of clinical trial software, streamlining processes, and reducing timelines. The increasing demand for improved patient engagement and decentralized clinical trials (DCTs) are also key factors driving market expansion. Smaller companies and CROs are increasingly adopting these technologies, creating a wider user base and fostering innovation. This dynamic market is characterized by continuous innovation, with vendors constantly enhancing their offerings to meet evolving needs and address emerging challenges in the clinical trial landscape. The market segmentation reveals a strong preference for cloud-based solutions over on-premise deployments.
Several factors are propelling the growth of the clinical trial software market. The escalating complexity of clinical trials, particularly those involving large datasets, diverse patient populations, and geographically dispersed sites, necessitates robust software solutions for efficient data management, analysis, and reporting. Regulatory requirements, such as Good Clinical Practice (GCP) guidelines and data privacy regulations like GDPR, are increasingly demanding, placing a premium on software that ensures compliance and data integrity. The rising cost of drug development necessitates optimizing trial timelines and reducing operational costs; clinical trial software is vital in achieving these objectives. The growth of decentralized clinical trials (DCTs), enabled by technological advancements, is another key driver. DCTs leverage technologies such as wearable sensors, telehealth platforms, and eConsent systems to enhance patient engagement and streamline data collection, creating a substantial demand for software capable of integrating and managing these diverse data sources. Furthermore, the increasing adoption of AI and machine learning in clinical trials is driving the need for sophisticated software solutions capable of handling and analyzing large and complex datasets, leading to more efficient and effective clinical research. The pursuit of faster time-to-market for new therapies further reinforces the urgency for efficient clinical trial processes supported by advanced software.
Despite the significant growth potential, the clinical trial software market faces several challenges. The high cost of implementation and maintenance of sophisticated software solutions can be a barrier for smaller organizations. Data security and privacy remain critical concerns, necessitating robust cybersecurity measures and compliance with stringent regulations. The integration of various software systems used within clinical trials can present technical challenges, requiring seamless data exchange and interoperability. The complexity of clinical trial designs and protocols can necessitate customization of software solutions, potentially increasing implementation time and costs. Furthermore, the need for ongoing training and support for users to effectively utilize the software can be an operational hurdle. The continuous evolution of technology and regulatory requirements necessitates ongoing updates and upgrades to software, representing an ongoing investment for organizations. The lack of standardization across various software systems and platforms can also impede data sharing and interoperability among different research teams and organizations. Finally, resistance to adopting new technologies within some organizations can hinder market growth.
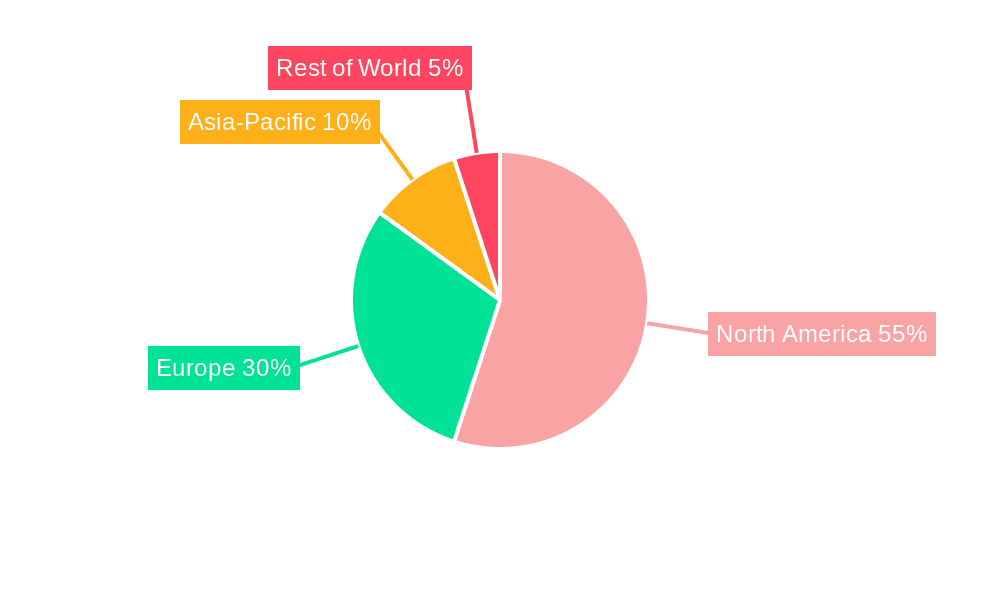
The North American market, particularly the United States, is expected to hold a dominant position in the clinical trial software market throughout the forecast period (2025-2033). This dominance is attributed to several factors:
Segment Domination: The cloud-based CTMS segment is expected to experience the most significant growth and ultimately dominate the market. This is driven by:
While pharmaceutical and biopharmaceutical companies remain the largest consumers, the adoption of clinical trial software among Clinical Research Organizations (CROs) is also experiencing significant growth, as they leverage these tools to provide more efficient and cost-effective services to their clients.
The confluence of technological advancements, regulatory pressures, and the increasing complexity of clinical trials is driving substantial growth in the clinical trial software market. The rising adoption of artificial intelligence and machine learning for data analysis and predictive modeling significantly enhances efficiency and reduces timelines. The growing demand for decentralized clinical trials (DCTs), which leverage technology to enhance patient engagement and simplify data collection, is another key catalyst. This expansion is fueled by a need for improved trial outcomes and reduced costs.
This report provides a comprehensive overview of the clinical trial software market, examining key trends, driving forces, challenges, and growth opportunities. It offers in-depth analysis of market segmentation by type (web-based, on-premise, cloud-based) and application (pharmaceutical companies, CROs, healthcare providers). The report includes detailed profiles of leading players in the industry, along with projections for market growth and revenue generation in the millions of dollars through 2033. It serves as a valuable resource for stakeholders in the clinical research industry, offering insights to inform strategic decision-making.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 13.7% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 13.7%.
Key companies in the market include Castor EDC, Clinical Conductor CTMS, Medrio, OpenClinica, ClinCapture, DFdiscover, ClinPlus CTMS, Dacima Clinical Suite, Smartsheet, Snappii, RealTime-CTMS, .
The market segments include Type, Application.
The market size is estimated to be USD 1017 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Clinical Trial Software," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Clinical Trial Software, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.