1. What is the projected Compound Annual Growth Rate (CAGR) of the Clinical Supply Chain Software?
The projected CAGR is approximately 11.2%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Clinical Supply Chain Software
Clinical Supply Chain SoftwareClinical Supply Chain Software by Type (Cloud-based, On-premise), by Application (Medicine, Medical Instruments, Diagnostic Reagents, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The global clinical supply chain software market is experiencing substantial expansion, driven by escalating clinical trial complexity and the imperative for enhanced efficiency and transparency in pharmaceutical and medical device supply management. Key growth accelerators include the widespread adoption of scalable, accessible cloud-based solutions, reducing IT overhead and appealing to diverse organizational sizes. The increasing demand for real-time supply chain visibility enables proactive risk mitigation and bolsters patient safety, critical in clinical trial environments. Technological integrations, such as AI and machine learning, are refining forecasting, optimizing inventory, and automating processes, further propelling market growth. While on-premise solutions currently hold a significant market share, cloud-based platforms are anticipated to grow at a faster rate due to their inherent advantages. Major application segments—medicines, medical instruments, and diagnostic reagents—are all showing growth, with medicines leading due to high trial volumes. Intense competition exists between established vendors and emerging tech providers. Geographic expansion, particularly in the Asia-Pacific region, presents considerable growth opportunities, notwithstanding challenges such as data security, regulatory compliance, and system integration.
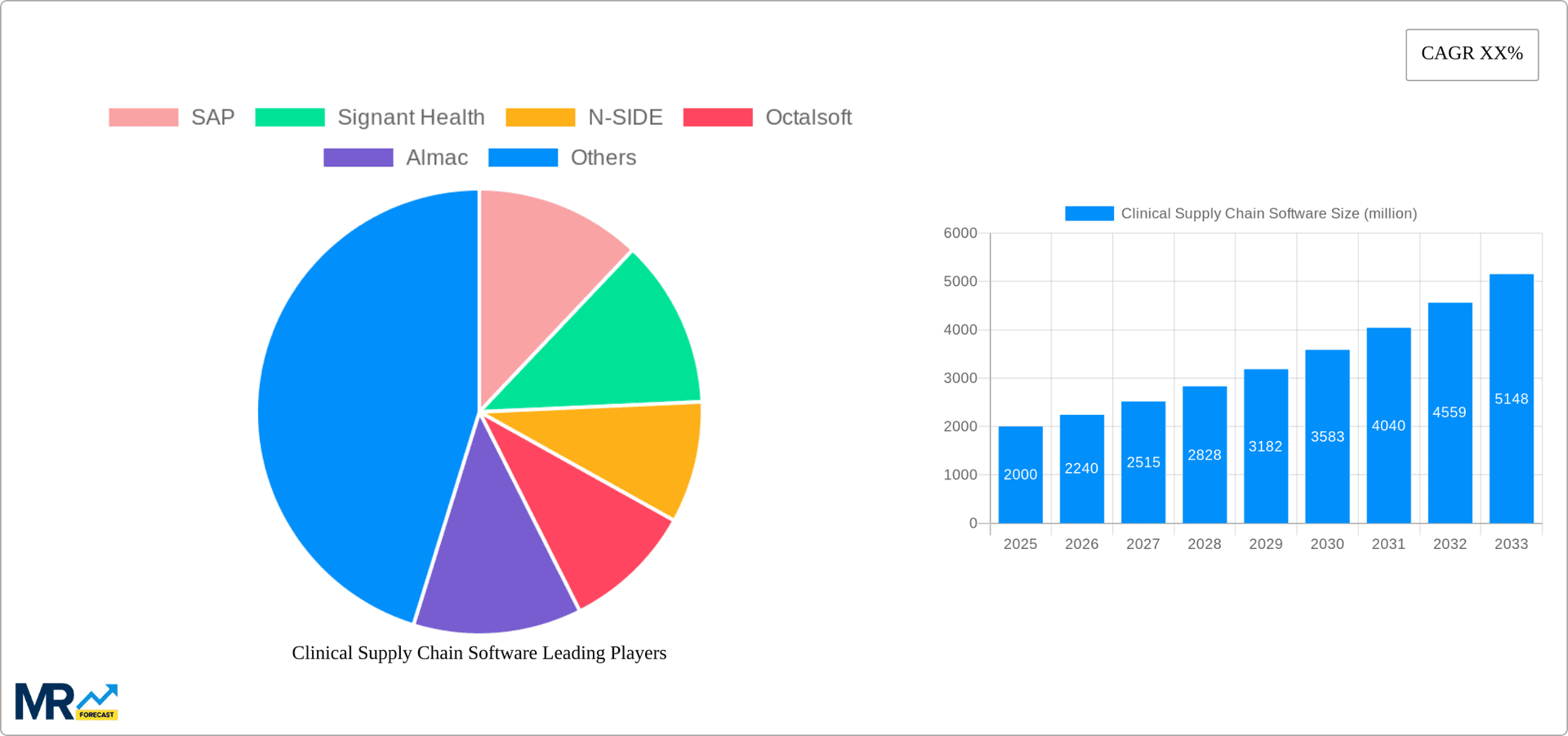

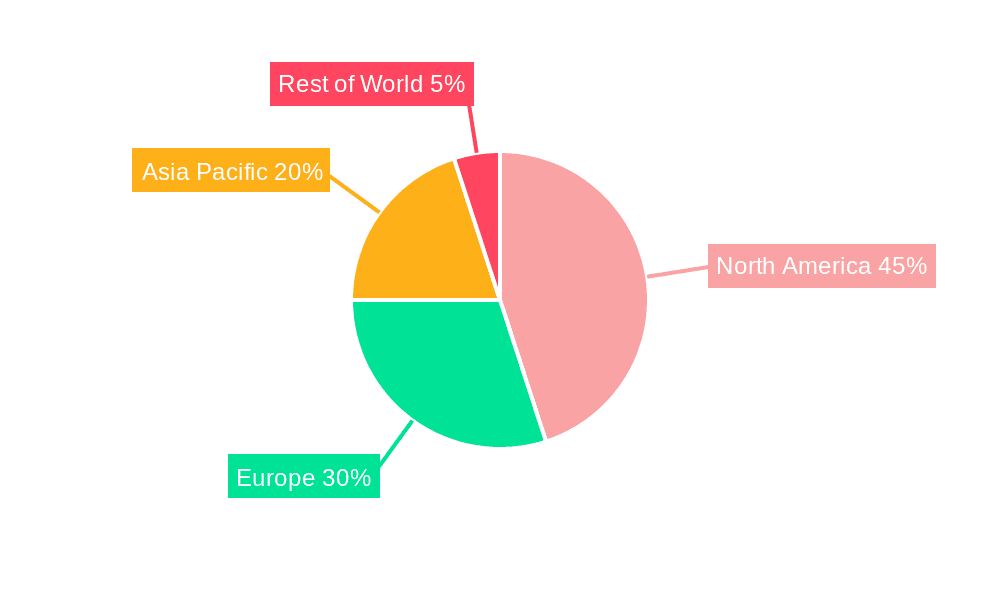
The market is projected to experience robust growth from 2025 to 2033. With a Compound Annual Growth Rate (CAGR) of 11.2% and an estimated market size of $1.46 billion in the base year 2025, the market is expected to surpass $4 billion by 2033. This trajectory will be fueled by continued technological innovation, rising clinical trial complexity, and stringent regulatory demands for enhanced traceability and transparency. Advanced analytics will facilitate proactive risk management and superior decision-making, conferring a competitive edge. Market segmentation is expected to evolve, with a growing emphasis on specialized solutions for specific therapeutic areas and trial designs, fostering diversification. North America is anticipated to maintain its leading market position, with significant contributions from Europe and Asia-Pacific.
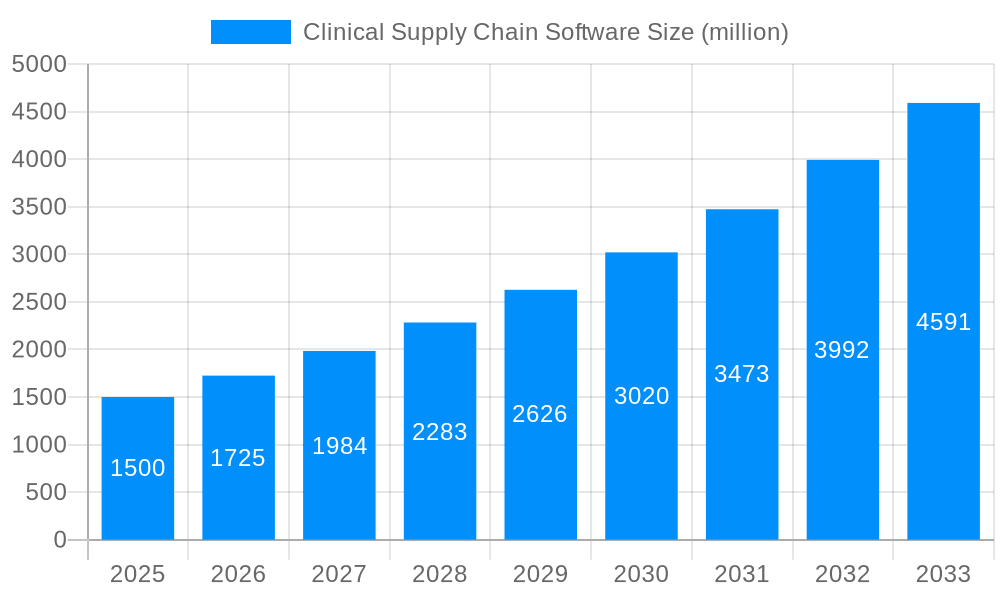

The clinical supply chain software market is experiencing robust growth, projected to reach multi-billion dollar valuations by 2033. Driven by increasing clinical trial complexity, stringent regulatory requirements, and the need for improved efficiency and transparency, the market is witnessing a significant shift towards advanced cloud-based solutions. The historical period (2019-2024) showed steady growth, laying the foundation for the exponential expansion anticipated during the forecast period (2025-2033). The estimated market value for 2025 (base year) is already substantial, reflecting the significant investments being made by pharmaceutical and biotechnology companies to optimize their supply chains. This trend is further fueled by the rising adoption of digital technologies, including AI and machine learning, which are enhancing visibility, traceability, and predictive capabilities within the clinical supply chain. The market is also seeing a rise in demand for specialized solutions tailored to specific therapeutic areas, such as oncology and rare diseases, reflecting the unique logistical challenges associated with these treatments. Furthermore, the increasing focus on patient centricity is driving the development of software solutions that improve patient engagement and adherence to clinical trial protocols. This overall trend points to a future where clinical supply chains are highly sophisticated, integrated, and data-driven, leading to faster drug development and ultimately better patient outcomes. The market's evolution is also marked by increasing mergers and acquisitions, as larger players seek to expand their market share and enhance their product offerings. This consolidation will likely lead to even greater efficiency and innovation in the coming years.
Several key factors are driving the expansion of the clinical supply chain software market. The ever-increasing complexity of clinical trials, involving diverse geographical locations, numerous investigational sites, and a wider range of investigational products, necessitates robust software solutions for effective management. Stringent regulatory compliance demands meticulous tracking and documentation of every stage of the supply chain, pushing companies towards software that ensures audit readiness and minimizes risk. The escalating costs associated with clinical trials are another significant driver. By improving efficiency, reducing waste, and enhancing visibility, clinical supply chain software contributes to significant cost savings. The need for real-time visibility and control across the entire supply chain is paramount for optimizing inventory management, minimizing delays, and preventing stockouts. This demand is fueled by the need for faster drug development and improved time-to-market. Furthermore, the growing adoption of advanced technologies, such as blockchain and AI, is enhancing the capabilities of these software solutions, creating opportunities for improved data analytics and predictive modeling. Finally, the increasing pressure to improve patient experience and enhance adherence to clinical trial protocols is prompting pharmaceutical companies to invest in software that facilitates better communication and collaboration across the entire supply chain, from manufacturing to patient delivery.
Despite the promising growth trajectory, the clinical supply chain software market faces several challenges. The high initial investment cost of implementing new software systems can be a significant barrier for smaller pharmaceutical companies or those with limited budgets. Integration with existing legacy systems can be complex and time-consuming, requiring significant IT resources and expertise. Data security and privacy concerns are also critical, given the sensitive nature of the data handled by these systems. Maintaining compliance with evolving regulatory requirements across different jurisdictions can be a considerable challenge, demanding continuous updates and adaptations to the software. The lack of skilled professionals proficient in managing and utilizing these sophisticated software solutions can hinder the efficient implementation and optimal utilization of the systems. Finally, the need for ongoing training and support for users can add to the overall cost and complexity of implementing and maintaining these systems. Addressing these challenges effectively is crucial for ensuring the continued growth and successful adoption of clinical supply chain software across the industry.
The cloud-based segment is poised to dominate the clinical supply chain software market. Its scalability, accessibility, and cost-effectiveness are attractive to companies of all sizes. Cloud-based solutions offer enhanced collaboration capabilities, real-time data visibility, and improved data security compared to on-premise solutions. The shift is driven by the increasing need for agile and flexible systems capable of adapting to the dynamic nature of clinical trials. Moreover, cloud-based systems often come with built-in functionalities for reporting and analytics, significantly improving decision-making processes.
The medicine application segment within clinical supply chain software commands a substantial market share. This reflects the critical role of efficient medicine supply chains in clinical trials. The complexity involved in managing various drug forms, dosages, and storage requirements necessitates robust software solutions. Moreover, the increasing focus on personalized medicine further enhances the importance of efficient medicine management.
The increasing adoption of advanced analytics and AI, combined with the growing demand for enhanced supply chain visibility and efficiency, is significantly boosting the growth of the clinical supply chain software industry. The ability to leverage data for predictive modeling, risk mitigation, and proactive decision-making is a key driver. Simultaneously, regulatory pressures for improved data integrity and traceability are pushing companies towards adopting more sophisticated software solutions. This convergence of technological advancements and regulatory compliance requirements is creating a favorable environment for rapid market expansion.
The clinical supply chain software market is poised for continued strong growth, driven by increasing complexities in clinical trials, regulatory pressures, and the demand for improved efficiency. Advanced analytics and AI are key catalysts, allowing for improved decision-making and risk mitigation. The market is shifting towards cloud-based solutions offering scalability and enhanced collaboration. North America and Europe remain key regions, but the Asia-Pacific region is witnessing significant growth. The medicine application segment dominates, reflecting the complexity of managing drug supply chains in clinical trials.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 11.2% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 11.2%.
Key companies in the market include SAP, Signant Health, N-SIDE, Octalsoft, Almac, UPS Healthcare, Clinigen, Yourway, Catalent, Parexel, Patheon, Covance, Endpoint Clinical, YPrime, .
The market segments include Type, Application.
The market size is estimated to be USD 1.46 billion as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in billion.
Yes, the market keyword associated with the report is "Clinical Supply Chain Software," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Clinical Supply Chain Software, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.