1. What is the projected Compound Annual Growth Rate (CAGR) of the Cell and Gene Therapy Supply Chain and Logistics?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Cell and Gene Therapy Supply Chain and Logistics
Cell and Gene Therapy Supply Chain and LogisticsCell and Gene Therapy Supply Chain and Logistics by Type (Ordering and Scheduling Services, Sample Collection Services, Logistics Services, Post Treatment Follow-Up Services, />Global Cell and Gene Therapy Supply Chain and Logistics Market, Segmentation By Application, Biobank, Cell Therapy Lab, Hospital, Research Institute), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The global cell and gene therapy supply chain and logistics market is experiencing robust growth, driven by the increasing adoption of advanced therapies and a surge in clinical trials. The market's expansion is fueled by several factors, including the rising prevalence of chronic diseases like cancer and genetic disorders, which necessitate innovative treatment options. Technological advancements in cell and gene therapies, coupled with supportive regulatory frameworks and increased investments in research and development, are further accelerating market growth. The market is segmented into various service types, including ordering and scheduling, sample collection, logistics, post-treatment follow-up, and encompassing diverse applications across biobanks, cell therapy labs, hospitals, and research institutions. Competition is intense, with established players like Thermo Fisher Scientific and McKesson alongside specialized firms like Biocair and Cryoport vying for market share. The North American region currently holds a significant share, due to advanced healthcare infrastructure and substantial investments in biotech. However, the Asia-Pacific region is expected to witness rapid growth in the coming years, driven by increasing healthcare expenditure and a growing patient population.

The market's growth trajectory is anticipated to continue its upward trend, with a substantial expansion projected over the forecast period. This growth will be influenced by several key factors, including the continuous development of novel cell and gene therapies, expansion of clinical trial activities, increasing demand for personalized medicine, and the development of more efficient and specialized logistics solutions for the sensitive nature of these therapies. However, challenges such as stringent regulatory requirements, high treatment costs, and the complexities involved in managing cold chain logistics for these highly sensitive biological materials remain as potential restraints to overall market expansion. Nevertheless, the long-term prospects for the cell and gene therapy supply chain and logistics market are extremely promising, driven by the potential to transform the treatment landscape for various life-threatening diseases. Successful market players will be those who effectively navigate the regulatory landscape, invest in cutting-edge technologies for transportation and storage, and strategically expand their global presence.
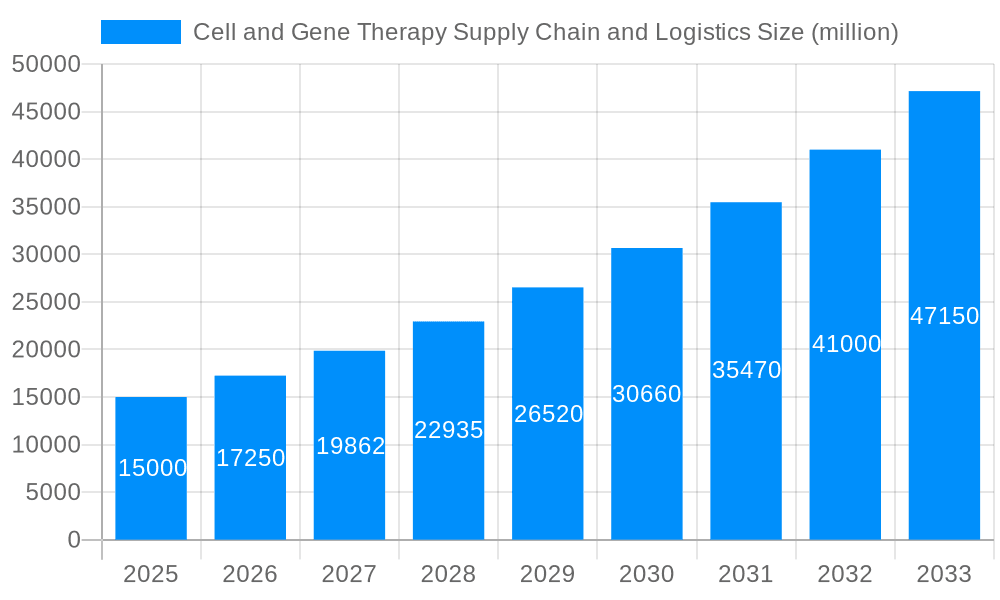

The global cell and gene therapy supply chain and logistics market is experiencing explosive growth, projected to reach XXX million units by 2033, up from XXX million units in 2025. This surge is driven by the increasing adoption of advanced therapies, a rising number of clinical trials, and the approval of novel cell and gene therapies. The market's evolution is characterized by a shift towards specialized services, demand for advanced technologies like real-time tracking and monitoring, and a heightened focus on regulatory compliance. The industry is consolidating, with larger players acquiring smaller companies to gain a competitive edge. This trend creates a more integrated and efficient supply chain, addressing the complex challenges posed by the unique characteristics of cell and gene therapies – namely their extreme temperature sensitivity, short shelf life, and individualized nature. We're also observing the rise of innovative solutions such as smart packaging, advanced cryopreservation techniques, and sophisticated data management systems to enhance safety, efficacy, and traceability throughout the entire supply chain. The industry is also witnessing a growing need for robust cold chain solutions, encompassing specialized transport vehicles and storage facilities capable of maintaining ultra-low temperatures. Furthermore, the increasing complexity of manufacturing processes for cell and gene therapies necessitates streamlined logistical systems to ensure seamless coordination between various stakeholders, from manufacturers to hospitals and patients. This intricate network necessitates detailed tracking, precise inventory management, and comprehensive documentation to meet rigorous regulatory demands. The market shows a strong inclination towards automation and digitization, impacting various operational aspects, from order management to patient data handling and logistics monitoring, contributing to improved efficiency, reduced operational costs, and enhanced patient safety.
Several factors are propelling the growth of the cell and gene therapy supply chain and logistics market. The expanding pipeline of cell and gene therapies undergoing clinical trials and receiving regulatory approvals is a primary driver. Increased investments in research and development are fueling innovation, leading to a greater variety of therapies available. The rising prevalence of chronic diseases such as cancer and genetic disorders, for which cell and gene therapies offer potential cures or effective treatments, significantly boosts demand. Furthermore, advancements in technology are contributing to the growth. Improved cryopreservation techniques, development of specialized transport containers, and real-time tracking systems enhance the safety and efficacy of these sensitive therapies. Stringent regulatory requirements are also acting as a catalyst, driving the demand for compliant logistics solutions and technology that ensure traceability and data integrity throughout the supply chain. The rising adoption of personalized medicine further contributes, necessitating customized supply chain solutions to meet the unique needs of individual patients. Finally, increased industry collaborations and partnerships between pharmaceutical companies, logistics providers, and technology developers are contributing to market expansion.
Despite its immense potential, the cell and gene therapy supply chain faces significant challenges. The extreme temperature sensitivity of these therapies necessitates robust and reliable cold chain management throughout the entire process, a complex and expensive undertaking. Maintaining the viability and potency of these therapies requires meticulous control over temperature, humidity, and other environmental factors, presenting significant logistical hurdles. The short shelf life of many cell and gene therapies adds another layer of complexity, demanding efficient and rapid transportation and handling. Furthermore, the highly individualized nature of cell and gene therapies necessitates precise inventory management and tailored logistical solutions for each patient, impacting costs and complexity. Regulatory compliance represents another major challenge. Strict guidelines governing the manufacturing, handling, and distribution of these therapies require thorough documentation, stringent quality control, and meticulous traceability. Ensuring data integrity and patient safety adds significant operational burdens and necessitates specialized technology investments. Finally, the lack of standardized protocols and processes across the industry hampers efficiency and interoperability, creating complexities in supply chain management.
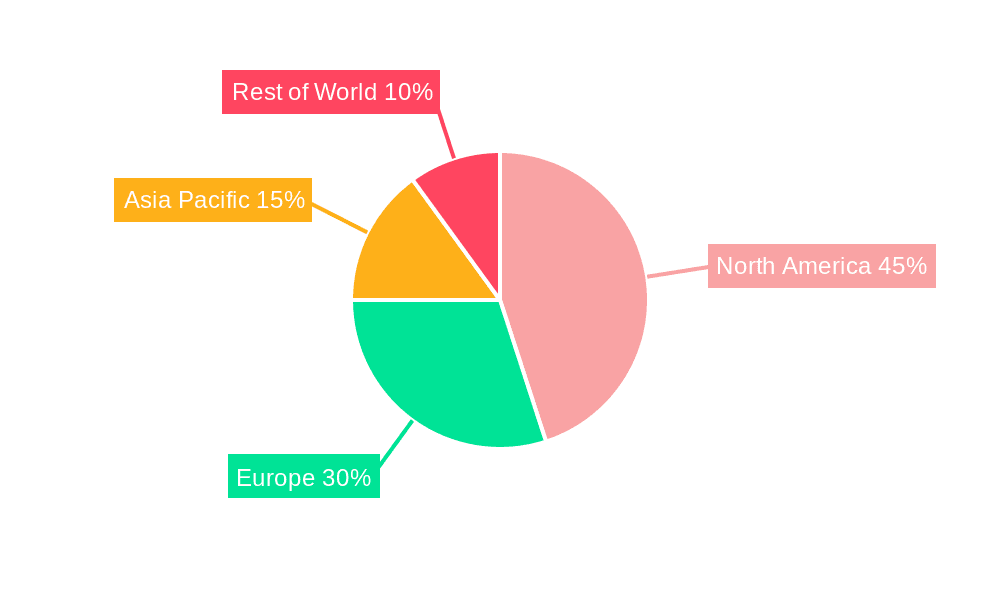
The North American market, particularly the United States, is expected to dominate the cell and gene therapy supply chain and logistics market due to the high concentration of clinical trials, regulatory approvals, and leading biopharmaceutical companies. Europe is another significant market player, with robust regulatory frameworks and increasing investments in advanced therapies. Asia-Pacific is poised for substantial growth, driven by expanding healthcare infrastructure and a growing demand for innovative treatments.
Dominant Segments:
Geographic Dominance:
The logistical complexity of cell and gene therapy necessitates a sophisticated supply chain that ensures the quality, safety and efficiency of these life-saving treatments. This aspect of advanced therapies often represents significant cost factors compared to other more traditional therapies.
The industry's growth is fueled by the increasing number of clinical trials and approvals for cell and gene therapies, coupled with technological advancements enhancing product handling and transportation, like smart packaging and automated systems. Government initiatives supporting advanced therapy development, combined with growing private investments and strategic partnerships within the industry further accelerate market growth. Finally, the rising prevalence of target diseases contributes significantly to this positive trend.
This report provides a comprehensive overview of the cell and gene therapy supply chain and logistics market, covering trends, drivers, challenges, key players, and future growth prospects. It offers detailed analysis across various segments, including logistics services, sample collection, and post-treatment follow-up, across different geographic regions. This report is a valuable resource for industry stakeholders seeking to understand this rapidly evolving market and navigate its complexities.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of XX% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
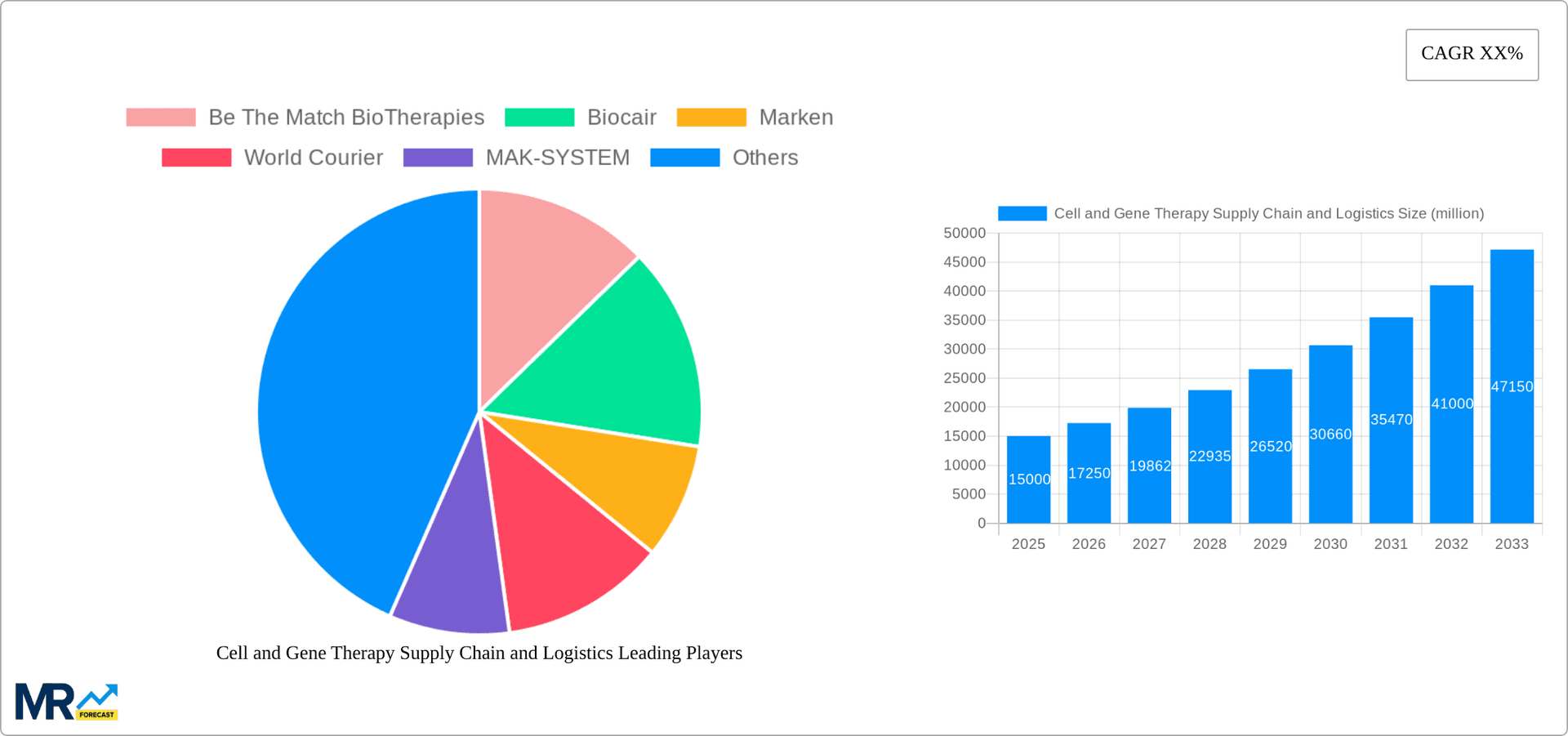
Key companies in the market include Be The Match BioTherapies, Biocair, Marken, World Courier, MAK-SYSTEM, Cryoport, Brooks Life Sciences, Clarkston Consulting, Hypertrust Patient Data Care, Haemonetics, MasterControl, TraceLink, SAVSU Technologies, TrakCel, Title21 Health Solutions, sedApta Group, Vineti, Stafa Cellular Therapy, Thermo Fisher Scientific, McKesson, BioLife Solutions, .
The market segments include Type.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Cell and Gene Therapy Supply Chain and Logistics," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Cell and Gene Therapy Supply Chain and Logistics, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.