1. What is the projected Compound Annual Growth Rate (CAGR) of the Wearable Low Frequency Pulse Anti-emetic Device?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Wearable Low Frequency Pulse Anti-emetic Device
Wearable Low Frequency Pulse Anti-emetic DeviceWearable Low Frequency Pulse Anti-emetic Device by Application (Medical Use, Household Use), by Type (Single Use, Multiple Use), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The global Wearable Low Frequency Pulse Anti-emetic Device market is poised for substantial growth, projected to reach an estimated market size of $1,800 million by 2025, with a compelling Compound Annual Growth Rate (CAGR) of 12.5% throughout the forecast period of 2025-2033. This upward trajectory is significantly driven by the increasing prevalence of conditions like chemotherapy-induced nausea and vomiting (CINV), motion sickness, and post-operative nausea and vomiting (PONV). The rising awareness among patients and healthcare providers regarding effective, non-pharmacological management of emesis, coupled with the inherent advantages of wearable devices – such as portability, user convenience, and reduced side effects compared to traditional anti-emetic drugs – are key catalysts for this expansion. The market's evolution is further supported by continuous technological advancements leading to more sophisticated and personalized device functionalities, enhancing efficacy and patient compliance.
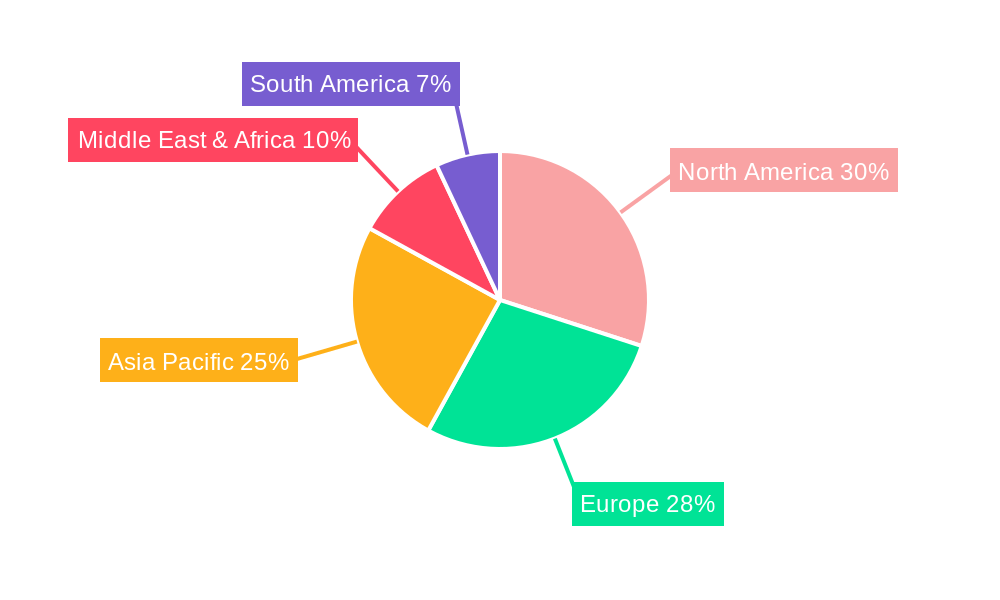
The market is segmented by application into Medical Use and Household Use, with Medical Use expected to dominate due to the critical need for effective nausea management in clinical settings. Within this, Single-Use devices are anticipated to hold a significant share, driven by stringent hygiene standards and the convenience offered in healthcare environments, though the Multiple-Use segment is gaining traction due to its cost-effectiveness and sustainability. Key players such as B Braun, ReliefBand, and EmeTerm are actively investing in research and development to innovate their product offerings and expand their market reach. Geographically, the Asia Pacific region, particularly China and India, presents a high-growth opportunity owing to a burgeoning patient population, increasing healthcare expenditure, and a growing adoption of advanced medical technologies. North America and Europe remain mature markets, characterized by high adoption rates and a strong focus on patient-centric care.
This report offers an in-depth analysis of the global Wearable Low Frequency Pulse Anti-emetic Device market, meticulously examining its trajectory from 2019 to 2033. The study leverages a robust methodology to provide invaluable insights for stakeholders, encompassing historical trends, current market dynamics, and future projections. Our comprehensive coverage will equip you with the knowledge to navigate this burgeoning sector, estimated to reach substantial figures in the millions by 2025 and beyond, with a keen focus on the forecast period of 2025-2033.
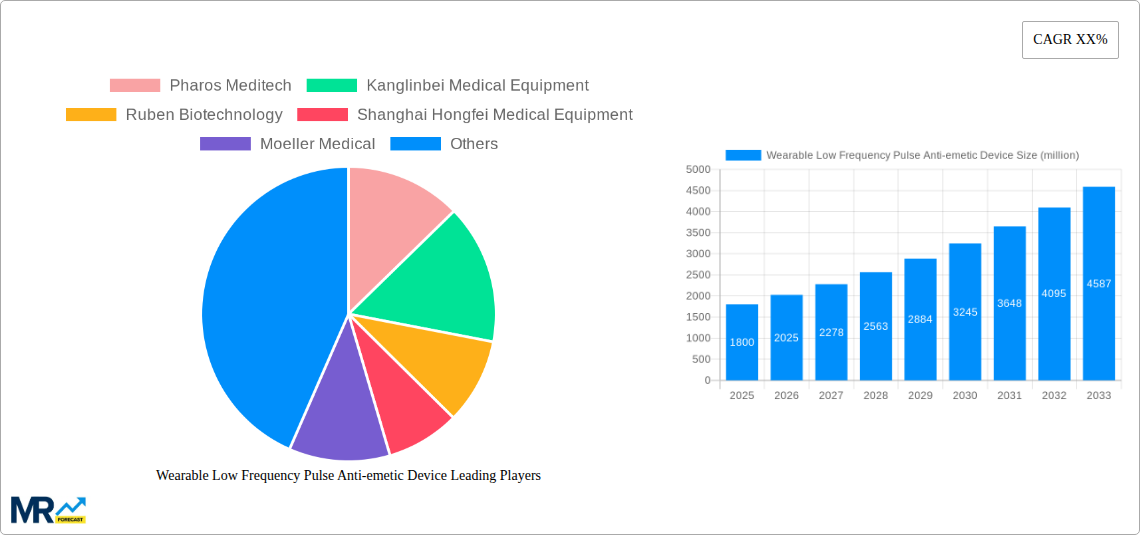
The Wearable Low Frequency Pulse Anti-emetic Device market is poised for significant expansion, driven by an increasing global prevalence of nausea and vomiting, coupled with a growing consumer preference for non-pharmacological and non-invasive therapeutic solutions. The Study Period of 2019-2033 will witness a progressive adoption of these devices across various applications. By the Base Year of 2025, the market is projected to have already established a strong foothold, with an estimated valuation reaching several million units. This growth trajectory is further amplified by advancements in wearable technology, leading to more discreet, comfortable, and user-friendly designs. The Forecast Period of 2025-2033 is expected to see exponential growth, driven by factors such as an aging global population, increased awareness of motion sickness and chemotherapy-induced nausea, and the growing demand for convenient home-use medical devices. The Estimated Year of 2025 will serve as a crucial pivot point, with sustained innovation and market penetration anticipated thereafter. Furthermore, the Historical Period of 2019-2024 laid the groundwork, demonstrating an initial interest and early adoption of these devices, characterized by iterative improvements in efficacy and user experience. The market's evolution will be marked by the diversification of product offerings to cater to a wider range of nausea-inducing conditions, from pregnancy-related morning sickness to post-operative recovery. The integration of smart features, such as personalized therapy settings and data tracking capabilities, will also play a pivotal role in shaping market trends, further enhancing user engagement and therapeutic outcomes, ultimately contributing to multi-million unit sales.
The rapid ascent of the Wearable Low Frequency Pulse Anti-emetic Device market is underpinned by a confluence of powerful driving forces. Foremost among these is the escalating global burden of conditions that trigger nausea and vomiting. This includes a rising incidence of motion sickness, a persistent challenge for travelers, and a growing number of individuals undergoing chemotherapy, a treatment known for its debilitating side effects of nausea. The aging demographic worldwide is also contributing significantly, as age-related conditions can often be accompanied by gastrointestinal discomfort. Equally impactful is the paradigm shift towards non-pharmacological and non-invasive medical interventions. Consumers are increasingly seeking alternatives to traditional medications, which can carry side effects, potential for drug interactions, and may not be suitable for all individuals, particularly pregnant women or those with certain pre-existing conditions. Wearable low-frequency pulse devices offer a safe, drug-free, and easily applicable solution. Moreover, continuous technological advancements in the wearable electronics sector are making these devices more sophisticated, comfortable, and aesthetically appealing. The miniaturization of components, improvements in battery life, and the development of intuitive user interfaces are all contributing to enhanced product adoption and market penetration, propelling the market into the multi-million unit sphere.
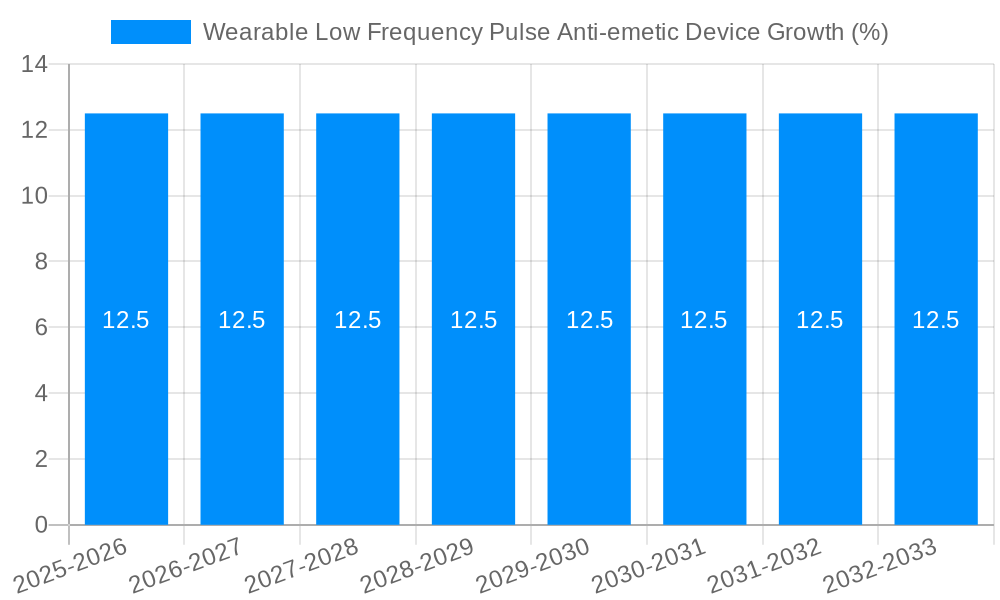
Despite its promising growth, the Wearable Low Frequency Pulse Anti-emetic Device market is not without its hurdles. A significant challenge lies in consumer awareness and education. Many potential users may be unaware of the existence or efficacy of these devices, mistaking them for novelties rather than legitimate therapeutic tools. Bridging this knowledge gap requires concerted marketing and educational efforts from manufacturers and healthcare providers. Furthermore, regulatory hurdles and the need for robust clinical validation can pose significant barriers to market entry and widespread adoption. Obtaining necessary certifications and demonstrating consistent, scientifically proven efficacy across diverse patient populations requires substantial investment in research and development. Perceived cost and accessibility, particularly for advanced models, could also limit adoption in certain demographic segments or geographical regions. While some devices are positioned as affordable alternatives to medication, the initial investment for higher-end models might be a deterrent. Finally, competition from established pharmaceutical treatments for nausea and vomiting, coupled with ingrained prescribing habits of healthcare professionals, presents a continuous challenge that requires innovative strategies to overcome.
The global Wearable Low Frequency Pulse Anti-emetic Device market is set to witness dominance in specific regions and segments, with North America and Europe emerging as key geographical players due to their advanced healthcare infrastructures, high disposable incomes, and a proactive approach to adopting innovative medical technologies. Within these regions, the Medical Use application segment is projected to be the dominant force.
Dominance of Medical Use Application:
Regional Dominance:
The synergy between the advanced healthcare infrastructure and the increasing demand for effective, non-pharmacological interventions for conditions like CINV and PONV, will propel the Medical Use application segment to command a significant share of the global Wearable Low Frequency Pulse Anti-emetic Device market, particularly within the leading regions of North America and Europe, reaching multi-million unit sales by the Estimated Year of 2025 and continuing its strong growth through the Forecast Period.
The growth of the Wearable Low Frequency Pulse Anti-emetic Device industry is being significantly catalyzed by several key factors. An increasing global prevalence of nausea-inducing conditions, from motion sickness to chemotherapy side effects, directly expands the potential user base. Furthermore, a pronounced shift towards non-pharmacological and non-invasive healthcare solutions empowers consumers to seek drug-free alternatives. Continuous technological advancements are leading to more sophisticated, user-friendly, and aesthetically pleasing devices, enhancing market appeal and adoption.
This report delves into a granular analysis of the Wearable Low Frequency Pulse Anti-emetic Device market, encompassing a comprehensive overview of its current landscape and future potential. It meticulously examines market size, growth rates, and key trends, providing readers with a data-driven understanding of the sector's dynamics. The report also offers a deep dive into the driving forces and challenges shaping the industry, offering strategic insights for stakeholders. Furthermore, it details the competitive landscape, highlighting the strategies and market positions of leading players. The comprehensive coverage ensures that all crucial aspects, from technological innovations to regional market penetrations, are addressed, providing an invaluable resource for informed decision-making.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Pharos Meditech, Kanglinbei Medical Equipment, Ruben Biotechnology, Shanghai Hongfei Medical Equipment, Moeller Medical, WAT Med, B Braun, ReliefBand, EmeTerm.
The market segments include Application, Type.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Wearable Low Frequency Pulse Anti-emetic Device," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Wearable Low Frequency Pulse Anti-emetic Device, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.