1. What is the projected Compound Annual Growth Rate (CAGR) of the U.S. Atrial Fibrillation Market?
The projected CAGR is approximately 3.9%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 U.S. Atrial Fibrillation Market
U.S. Atrial Fibrillation MarketU.S. Atrial Fibrillation Market by Drug Class (Anticoagulants, Anti-arrhythmic Drugs), by Route of Administration (Oral, Intravenous, Others), by Distribution Channel (Hospital Pharmacies, Online Pharmacies, Retail Pharmacies), by U.S. Forecast 2026-2034
The size of the U.S. Atrial Fibrillation Market was valued at USD 5.51 USD Billion in 2023 and is projected to reach USD 7.20 USD Billion by 2032, with an expected CAGR of 3.9% during the forecast period. Atrial fibrillation (AFib) is the most common type of arrhythmia in the United States, characterized by an irregular and often rapid heart rhythm. It occurs when the heart’s upper chambers (atria) beat chaotically, out of sync with the lower chambers (ventricles). AFib increases the risk of stroke, heart failure, and other cardiovascular complications, making it a critical public health concern.
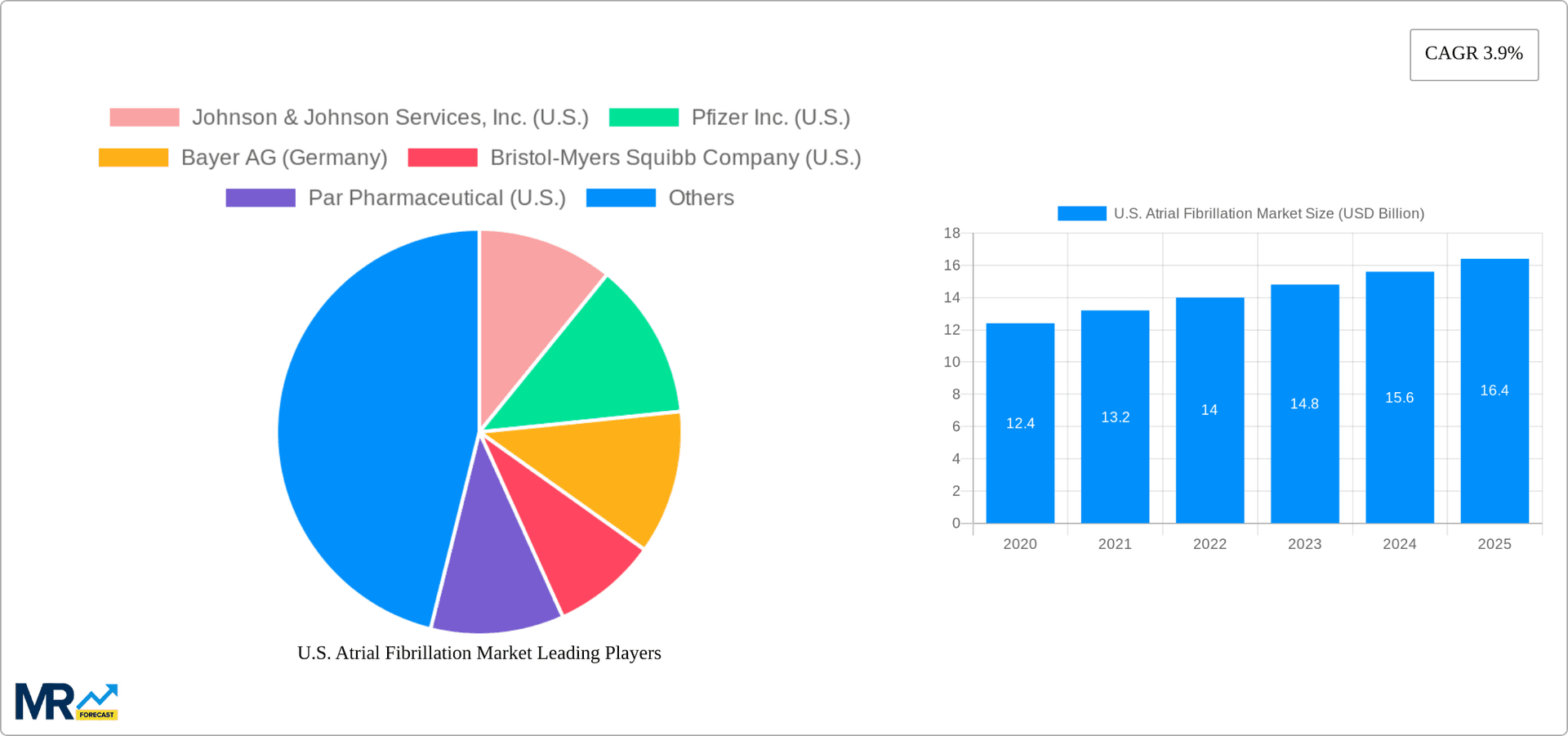

The U.S. Atrial Fibrillation market is experiencing significant growth driven by a confluence of factors. A primary catalyst is the increasing incidence and prevalence of atrial fibrillation (AFib), a complex cardiac arrhythmia often associated with aging populations and comorbidities like hypertension, diabetes, and obesity. Complementing this is the rapid adoption of advanced diagnostic techniques, including sophisticated electrocardiograms (ECGs), Holter monitors, implantable loop recorders, and 3D electroanatomical mapping systems. These tools enable earlier and more accurate detection, leading to improved patient management and outcomes. Furthermore, enhanced awareness campaigns targeting both healthcare professionals and the general public are empowering individuals to recognize AFib symptoms and seek timely medical attention. This proactive approach is crucial for initiating early interventions and mitigating the risk of debilitating complications such as stroke and heart failure. The market is also being shaped by continuous innovation in therapeutic interventions, with the development and approval of novel oral anticoagulants (NOACs/DOACs) and cutting-edge anti-arrhythmic medications that offer improved efficacy, safety profiles, and patient convenience compared to older therapies.


The rising prevalence of cardiovascular diseases, including hypertension, obesity, and diabetes, is a major factor fueling the growth of the U.S. Atrial Fibrillation Market. These conditions increase the risk of developing atrial fibrillation, thereby driving demand for diagnosis and treatment services. Technological advancements in the field of electrophysiology have led to the development of innovative ablation techniques, such as catheter ablation, which offer minimally invasive and effective treatment options.
The high cost of treatment, including hospitalizations, medications, and follow-up care, poses a challenge to the growth of the market. Additionally, the potential side effects associated with anticoagulants and anti-arrhythmic drugs can limit their use in certain patient populations. Moreover, the complexity of managing atrial fibrillation, which often requires a multidisciplinary approach involving cardiologists, electrophysiologists, and primary care physicians, can also present challenges.
The United States unequivocally leads the global atrial fibrillation market. This dominance is underpinned by a robust healthcare infrastructure characterized by high per capita healthcare spending, widespread access to advanced medical technologies, and a high burden of cardiovascular diseases. The nation's strong commitment to pioneering medical research and development further fuels innovation and the adoption of novel treatments. Within this dynamic market, the anticoagulant segment is poised for continued dominance. This is largely attributed to the critical role anticoagulants play in the primary prevention of stroke and thromboembolic events in AFib patients, a significant concern given the condition's inherent risks. The shift towards oral anticoagulants, particularly Direct Oral Anticoagulants (DOACs), is a defining trend within this segment. Their perceived advantages in terms of predictable pharmacokinetics, reduced need for routine monitoring, and fewer drug and food interactions make them the preferred choice for many patients and prescribers, ensuring the oral administration route remains the cornerstone of AFib anticoagulation therapy.
The growing adoption of wearable devices, such as smartwatch-based electrocardiograms, is expected to contribute to market growth by enabling early detection and remote monitoring of atrial fibrillation. The increasing focus on personalized medicine and the development of tailored treatment plans based on individual patient characteristics are also driving market growth. Furthermore, the emergence of telemedicine platforms for remote patient monitoring and consultation is enhancing access to specialized care, particularly in underserved areas.
This comprehensive report offers an in-depth and granular analysis of the U.S. Atrial Fibrillation market, meticulously dissecting its intricate dynamics, emerging industry trends, key growth drivers, persistent challenges, and the competitive landscape. The report provides a detailed market segmentation strategy, encompassing treatment modalities, diagnostic tools, and end-user demographics. A thorough regional analysis offers insights into variations across different states and healthcare systems within the U.S. Projections for market growth are presented with robust forecasting methodologies, accompanied by strategic insights into the key market players, their product portfolios, and their competitive strategies. Furthermore, the report delivers a comprehensive assessment of current and emerging technologies, including advancements in remote monitoring and AI-driven diagnostic tools, alongside an examination of the evolving regulatory policies that shape market access and innovation. It also highlights significant market opportunities for stakeholders looking to capitalize on the evolving needs of patients and healthcare providers in the U.S. Atrial Fibrillation landscape.
The DROCT (Direct Resellers Only, Credit Terms Negotiable) model is commonly used in the pharmaceutical industry.
The pricing of atrial fibrillation drugs and treatments varies widely depending on factors such as the type of medication, dosage, duration of treatment, and healthcare provider.
The U.S. imports a significant portion of its atrial fibrillation drugs and medical devices from countries such as China, India, and Germany, while also exporting these products to other countries around the world.
Major pharmaceutical companies hold patents and trademarks related to atrial fibrillation drugs, diagnostic tools, and treatment devices, providing them with exclusivity over their products for a period of time.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 3.9% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 3.9%.
Key companies in the market include Johnson & Johnson Services, Inc. (U.S.), Pfizer Inc. (U.S.), Bayer AG (Germany), Bristol-Myers Squibb Company (U.S.), Par Pharmaceutical (U.S.), Sanofi (France), DAIICHI SANKYO COMPANY, LIMITED (Japan), Boehringer Ingelheim International GmbH (Germany).
The market segments include Drug Class, Route of Administration, Distribution Channel.
The market size is estimated to be USD 5.51 USD Billion as of 2022.
Increasing Burden of Geriatric Population Coupled with Rising Hypertension to Augment Market Growth.
Increasing Burden of Geriatric Population Coupled with Rising Hypertension to Augment Market Growth.
Increasing Burden of Geriatric Population Coupled with Rising Hypertension to Augment Market Growth.
October 2023: Milestone Pharmaceuticals announced the submission of a new drug application (NDA) to the U.S. FDA for its drug etripamil, a calcium channel blocker intended for the treatment of patients suffering from AFib.
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 2850, USD 3850, and USD 4850 respectively.
The market size is provided in terms of value, measured in USD Billion.
Yes, the market keyword associated with the report is "U.S. Atrial Fibrillation Market," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the U.S. Atrial Fibrillation Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.