1. What is the projected Compound Annual Growth Rate (CAGR) of the Spinal Cord Injury Care Devices?
The projected CAGR is approximately 4.8%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Spinal Cord Injury Care Devices
Spinal Cord Injury Care DevicesSpinal Cord Injury Care Devices by Type (Stimulation Devices, Assistive Devices, Mobility Device, Others, World Spinal Cord Injury Care Devices Production ), by Application (Rehabilitation Centres, Assistive Living Facilities, Long-term Care Centers, Home Care Settings, World Spinal Cord Injury Care Devices Production ), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The global spinal cord injury (SCI) care devices market is experiencing robust growth, driven by increasing prevalence of SCI, technological advancements in assistive and stimulation devices, and rising demand for improved rehabilitation outcomes. The market, currently valued at approximately $4 billion (estimated based on typical market sizes for similar medical device sectors and growth rates), is projected to exhibit a healthy Compound Annual Growth Rate (CAGR) of around 7-8% from 2025 to 2033. This expansion is fueled by several key factors. Firstly, the aging global population contributes to a higher incidence of SCI due to age-related factors like falls and degenerative diseases. Secondly, ongoing research and development efforts are constantly improving the functionality and accessibility of SCI care devices, leading to enhanced patient mobility, independence, and overall quality of life. This includes advancements in exoskeletons, robotic-assisted therapy, and implantable stimulation devices. Finally, increased awareness among healthcare professionals and patients about the availability and effectiveness of these devices is driving greater adoption rates, particularly in developed nations with robust healthcare infrastructure. The market is segmented across various device types (stimulation, assistive, mobility), application settings (rehabilitation centers, home care), and geographical regions. North America and Europe currently hold a significant market share, owing to higher healthcare expenditure, advanced medical technology adoption, and a large patient pool. However, emerging economies in Asia-Pacific are expected to show substantial growth in the coming years due to rising healthcare investments and expanding awareness. Challenges remain, including the high cost of these advanced devices, limited insurance coverage in some regions, and the need for extensive training to effectively utilize the technologies. Nevertheless, the long-term outlook for the SCI care devices market remains positive, with opportunities for continued innovation and market expansion across various segments and geographies.
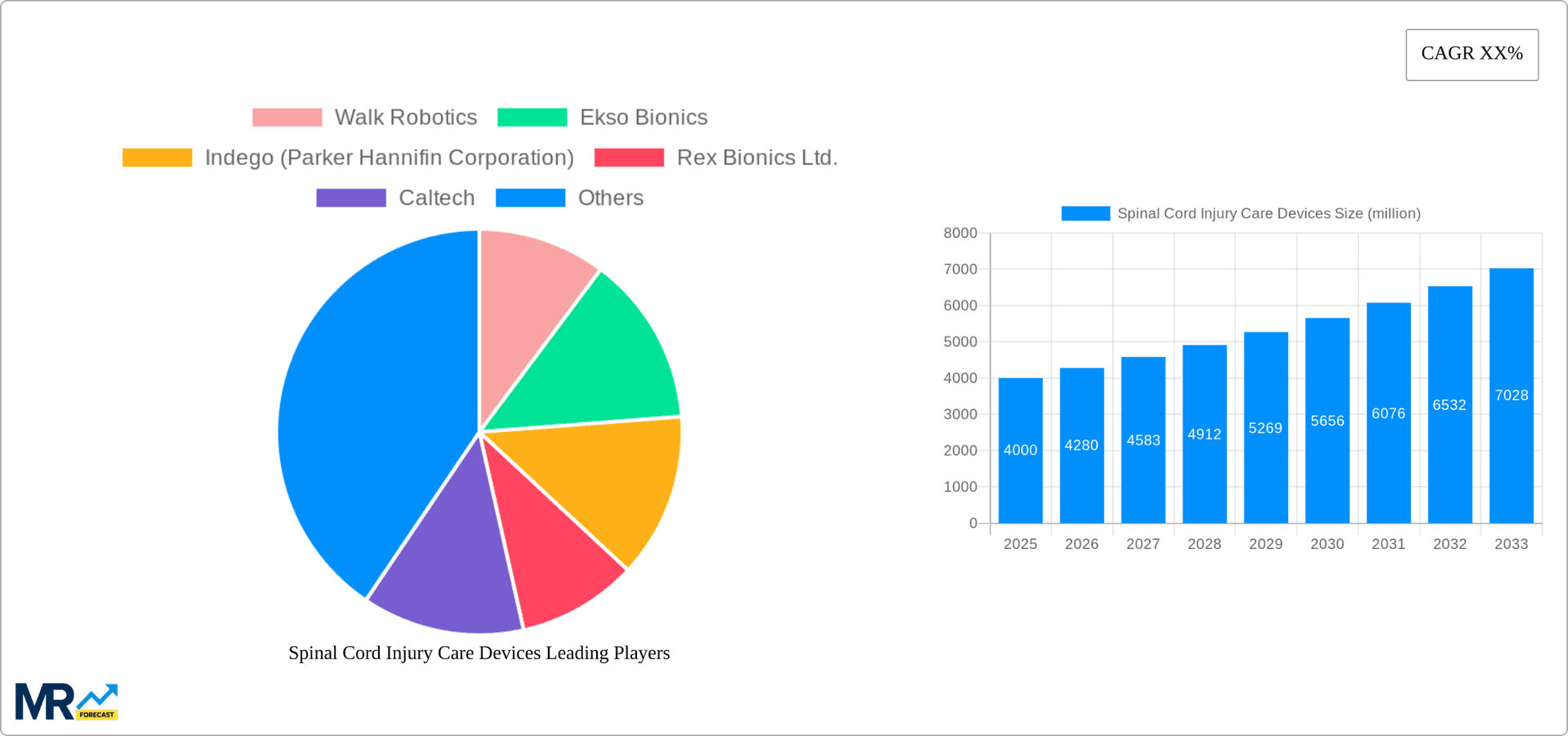

The competitive landscape is marked by a blend of established medical device companies and specialized robotics firms. Key players are focusing on strategic collaborations, acquisitions, and product development to gain a competitive edge. While established players like Medtronic and Boston Scientific leverage their existing distribution networks and brand recognition, innovative startups are contributing disruptive technologies. This dynamic competitive environment is driving further market expansion through diversification of product offerings, improved device accessibility, and intensified research into innovative treatment modalities. The increasing focus on personalized medicine and the integration of artificial intelligence in rehabilitation therapies present lucrative avenues for future growth. Furthermore, the increasing prevalence of telehealth and remote patient monitoring solutions is likely to further impact market expansion, improving access to care, particularly in remote and underserved areas.
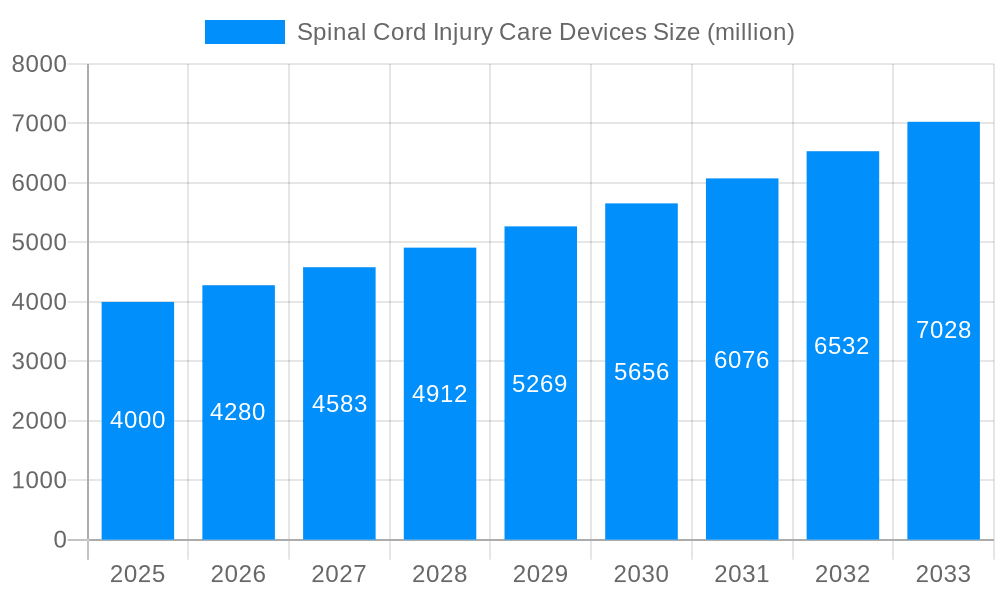

The global spinal cord injury (SCI) care devices market is experiencing robust growth, driven by increasing prevalence of SCI, technological advancements leading to more sophisticated and effective devices, and rising awareness about available treatment options. The market, valued at approximately $X billion in 2025, is projected to reach $Y billion by 2033, exhibiting a Compound Annual Growth Rate (CAGR) of Z%. This growth is fueled by a combination of factors, including the aging global population, increased incidence of traumatic injuries (due to accidents and violence), and an expanding understanding of the long-term consequences of SCI, leading to greater demand for improved quality of life solutions. The market is witnessing a shift towards minimally invasive procedures and personalized medicine approaches, reflecting the need for less disruptive treatments and tailored solutions for individual patient needs. Furthermore, the integration of advanced technologies like artificial intelligence (AI) and machine learning (ML) is enhancing the precision and effectiveness of SCI care devices. This trend is evident in the development of smart assistive devices, which offer personalized feedback and adaptive assistance to patients, significantly improving their rehabilitation outcomes. The market shows a clear preference for devices offering improved mobility, reduced pain, and enhanced functional independence, pushing manufacturers to constantly innovate and develop more advanced solutions. This positive market trajectory is expected to continue as advancements in neurotechnology and rehabilitation techniques continue to emerge.
Several key factors are propelling the growth of the spinal cord injury care devices market. The rising prevalence of spinal cord injuries worldwide, due to factors such as road accidents, sports injuries, and violence, is a significant driver. The aging global population also contributes to increased incidence of SCI, particularly due to age-related degenerative conditions. Technological advancements are crucial, with the development of sophisticated devices like implantable stimulators, exoskeletons, and advanced wheelchairs enhancing the quality of life for SCI patients. Increased healthcare spending and improved healthcare infrastructure in developing economies are also contributing factors. A growing awareness among patients and healthcare professionals about the benefits of these devices and the availability of better rehabilitation programs further boosts market growth. Government initiatives aimed at supporting research and development in this area, along with favorable reimbursement policies, further incentivize market expansion. Finally, the increasing demand for home-based care and assistive technologies is contributing to the growth of the SCI care devices market as patients seek greater independence and mobility in familiar environments.
Despite the significant growth potential, the spinal cord injury care devices market faces several challenges. High costs associated with these devices, including purchase price, maintenance, and rehabilitation therapies, can significantly limit access, particularly in low- and middle-income countries. The complexity of SCI and the variability of patient needs often lead to limited device efficacy and personalization difficulties. Regulatory hurdles and the lengthy approval processes for new devices can also hinder market entry. Furthermore, the long-term effectiveness and durability of some devices remain concerns for both patients and healthcare providers. The need for skilled professionals for device operation, maintenance, and patient training presents another obstacle, particularly in resource-constrained settings. Finally, the potential for adverse effects or complications associated with the use of some devices can influence patient adoption and create a barrier to market growth. Addressing these challenges is crucial for ensuring wider access and better outcomes for individuals with SCI.
Dominant Segments:
Mobility Devices: This segment is projected to hold the largest market share throughout the forecast period (2025-2033). The increasing demand for improved mobility and independence among SCI patients fuels this significant growth. This includes advanced wheelchairs, exoskeletons, and other assistive devices designed to enhance mobility and participation in daily life activities. The innovation in this segment is particularly strong, with manufacturers continuously developing lighter, more comfortable, and technologically advanced mobility aids.
Rehabilitation Centers: Rehabilitation centers represent a major application segment, driving the demand for a wide range of SCI care devices. These centers require a comprehensive array of devices for diagnosis, treatment, and rehabilitation, contributing significantly to overall market growth. The concentrated nature of these facilities makes them an attractive target for manufacturers, leading to competitive market dynamics and continuous innovation.
Dominant Regions/Countries:
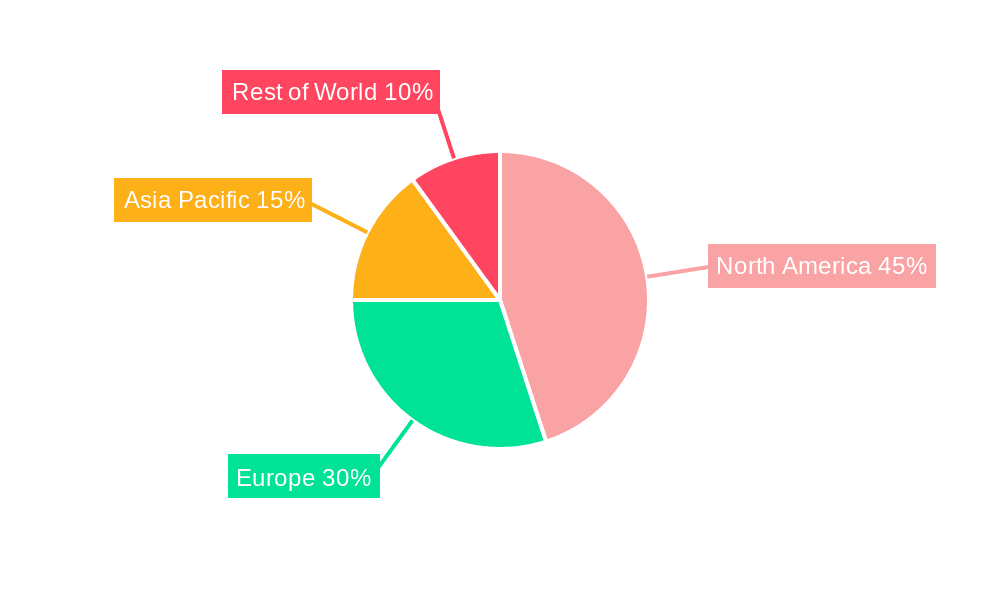
North America: The North American market is expected to maintain its leading position due to high healthcare expenditure, advanced medical infrastructure, and a relatively high prevalence of SCI. The presence of key players and robust research & development activities in the region further strengthen its dominance.
Europe: Europe's well-established healthcare systems and substantial investments in medical technology research and development contribute to its strong market performance. The increasing awareness about SCI care and the adoption of advanced devices drive growth in this region.
Asia-Pacific: While currently smaller than North America and Europe, the Asia-Pacific region is demonstrating rapid growth. This is propelled by rising healthcare expenditure, an increasing aging population, and a growing awareness of SCI care options. The region is predicted to exhibit the highest CAGR during the forecast period due to the rapid economic expansion and rising disposable incomes.
The market growth in these regions is driven by factors such as increasing prevalence of SCI, rising healthcare expenditure, technological advancements, and favorable government regulations. The continued focus on improving the quality of life for individuals with SCI will continue to drive demand and innovation within these key market segments and geographical areas.
The spinal cord injury care devices industry is experiencing significant growth due to several key catalysts. These include advancements in neurotechnology, leading to the development of more sophisticated and effective devices; a rising awareness and understanding of SCI, leading to increased patient demand for improved treatment options; growing healthcare expenditure and insurance coverage facilitating access to advanced devices; and government initiatives and research funding promoting innovation in this sector. The increasing focus on personalized medicine and the integration of smart technologies are also accelerating market growth, with the potential for significant improvements in patient outcomes and quality of life.
This report provides a comprehensive overview of the spinal cord injury care devices market, analyzing market trends, drivers, challenges, key players, and significant developments. It offers detailed insights into various segments, including device types, application areas, and geographical regions, providing valuable information for stakeholders interested in understanding and participating in this rapidly evolving market. The report combines qualitative and quantitative analysis, incorporating data from various sources to deliver a well-rounded picture of the market landscape and future growth potential.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 4.8% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 4.8%.
Key companies in the market include Walk Robotics, Ekso Bionics, Indego (Parker Hannifin Corporation), Rex Bionics Ltd., Caltech, Össur, Steeper, Inc., Boston Scientific corporation, Medtronic Plc., Ottobock, RGK Wheelchairs, Rifton Equipment, Allard USA, Inc., Fillauer LLC, College Park Industries, Mobius Bionics., Parker Hannifin, Nevro, ProBed Medical Technologies Inc., BLUE CHIP MEDICAL, Restorative Therapies, MYOLYN, LLC.
The market segments include Type, Application.
The market size is estimated to be USD XXX N/A as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in N/A and volume, measured in K.
Yes, the market keyword associated with the report is "Spinal Cord Injury Care Devices," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Spinal Cord Injury Care Devices, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.