1. What is the projected Compound Annual Growth Rate (CAGR) of the RNAi for Therapeutic?
The projected CAGR is approximately 41.2%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 RNAi for Therapeutic
RNAi for TherapeuticRNAi for Therapeutic by Type (siRNA, miRNA, shRNA), by Application (Cancer, Cardiovascular, HBV, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
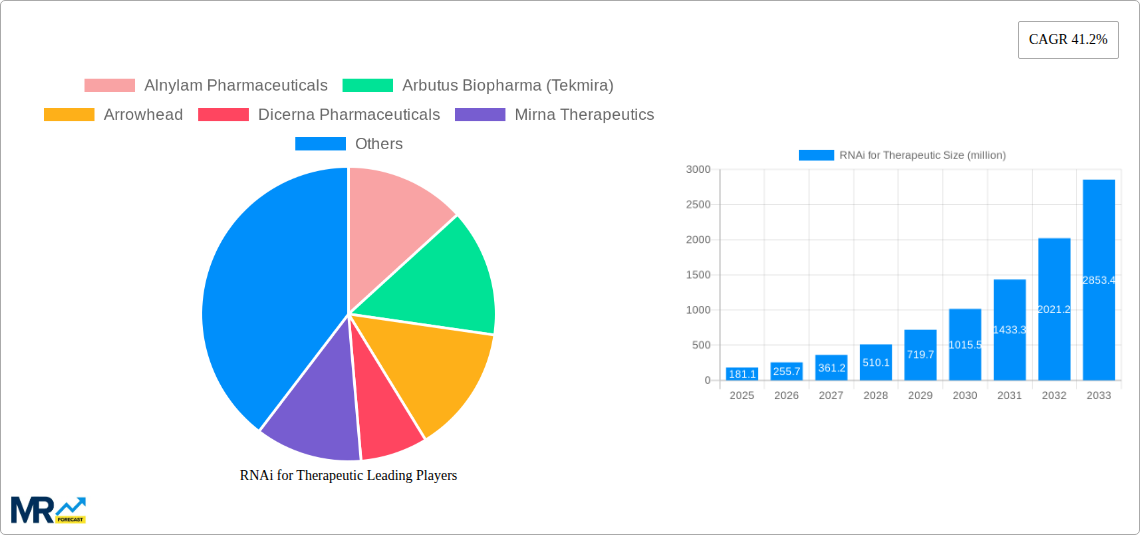
The RNA interference (RNAi) therapeutics market is poised for explosive growth, projected to reach \$181.1 million in the base year 2025 with an astonishing Compound Annual Growth Rate (CAGR) of 41.2% through 2033. This surge is fueled by the increasing understanding of gene regulation and the development of sophisticated delivery mechanisms, enabling RNAi to target diseases at their genetic roots. The market is witnessing robust advancements in therapeutic modalities, with Small Interfering RNA (siRNA) currently dominating due to its established efficacy and pipeline. However, microRNA (miRNA) and short hairpin RNA (shRNA) segments are rapidly gaining traction, offering diverse therapeutic possibilities. The application landscape is primarily driven by the significant unmet needs and promising clinical trial outcomes in cancer treatment. Beyond oncology, burgeoning research and development efforts are expanding RNAi's reach into cardiovascular diseases, Hepatitis B virus (HBV) infections, and a spectrum of other rare genetic disorders. This broad applicability, coupled with a growing pipeline of investigational therapies, underpins the sustained high growth trajectory.
Key players like Alnylam Pharmaceuticals, Arbutus Biopharma, and Arrowhead Pharmaceuticals are at the forefront of innovation, investing heavily in research and development to overcome delivery challenges and enhance therapeutic profiles. Strategic collaborations and mergers are also shaping the competitive landscape, accelerating the commercialization of novel RNAi-based drugs. Emerging trends include the development of targeted delivery systems for improved specificity and reduced off-target effects, alongside advancements in next-generation RNAi technologies offering enhanced stability and potency. While the market presents immense opportunities, challenges such as the high cost of therapy, complex manufacturing processes, and the need for extensive clinical validation continue to be addressed by industry stakeholders. The substantial investment in research, coupled with a growing number of clinical approvals and positive trial data, strongly indicates that RNAi therapeutics will play a pivotal role in precision medicine for years to come.
This report provides an in-depth analysis of the RNA interference (RNAi) for therapeutic market, spanning a comprehensive study period from 2019 to 2033. Utilizing 2025 as the base and estimated year, and focusing on the forecast period of 2025-2033, the report meticulously examines historical trends from 2019-2024. The global market is projected to witness significant expansion, with revenues expected to reach over $7,500 million by the end of the forecast period. This valuation underscores the transformative potential of RNAi-based therapies in addressing unmet medical needs across a spectrum of diseases. The report delves into the intricate dynamics of this rapidly evolving sector, offering actionable insights for stakeholders.
The RNAi for therapeutic landscape is characterized by a dynamic interplay of groundbreaking scientific advancements and increasing clinical validation, driving substantial market growth. XXX The market is experiencing a paradigm shift, moving from niche applications to broader therapeutic indications. The successful development and commercialization of several RNAi-based drugs have significantly boosted investor confidence and accelerated research and development efforts across the industry. Specifically, small interfering RNA (siRNA) technologies have emerged as frontrunners, accounting for a substantial portion of the market share due to their established efficacy and robust delivery mechanisms. Furthermore, advancements in delivery systems, including lipid nanoparticles (LNPs) and viral vectors, are overcoming previous hurdles, enabling more targeted and efficient delivery of RNAi molecules to disease sites. This technological progress is critical for enhancing therapeutic outcomes and expanding the applicability of RNAi across a wider range of diseases. The increasing prevalence of chronic conditions like cardiovascular diseases and viral infections, such as Hepatitis B Virus (HBV), presents a fertile ground for RNAi therapeutics, with significant market potential. The industry is also witnessing a growing interest in developing RNAi therapies for rare genetic disorders, which often have limited treatment options. The regulatory landscape is becoming more favorable, with increased clarity and streamlined approval pathways for RNAi-based drugs, further stimulating market expansion. The collaborative efforts between pharmaceutical giants and innovative biotech companies are also a key trend, fostering innovation and accelerating the translation of research into clinical practice. The market is projected to grow at a Compound Annual Growth Rate (CAGR) exceeding 18% during the forecast period, indicating sustained and robust expansion. This growth is fueled by an expanding pipeline of RNAi candidates and the growing acceptance of these novel therapeutic modalities by both healthcare providers and patients. The increasing focus on personalized medicine further aligns with the potential of RNAi to silence specific disease-causing genes, offering highly targeted treatments.
Several powerful forces are propelling the RNAi for therapeutic market forward, creating an environment ripe for innovation and growth. The primary driver is the inherent precision of RNAi technology, which allows for the targeted silencing of specific genes responsible for disease pathogenesis. This gene-specific mechanism offers a level of therapeutic specificity that is often unmatched by traditional small molecules or biologics, leading to potentially improved efficacy and reduced off-target side effects. The increasing understanding of the genetic underpinnings of various diseases, coupled with advancements in genomics and bioinformatics, has identified a multitude of potential gene targets amenable to RNAi intervention. Furthermore, the successful clinical development and commercialization of several RNAi therapies for previously intractable conditions have significantly de-risked the technology and boosted confidence among investors, researchers, and regulatory bodies. This has translated into substantial investments flowing into the sector, fueling further research and development. The growing prevalence of chronic and genetic diseases, such as cardiovascular disorders, HBV, and certain cancers, presents a significant unmet medical need that RNAi therapies are well-positioned to address. The aging global population also contributes to the rising burden of these diseases, thereby expanding the patient pool and market opportunity for effective RNAi treatments. Beyond these core drivers, ongoing innovations in RNAi delivery systems, including advancements in lipid nanoparticles, viral vectors, and novel conjugate chemistries, are crucial in overcoming the biological barriers to effective cellular uptake and systemic distribution, thereby broadening the therapeutic potential of RNAi.
Despite the immense promise, the RNAi for therapeutic market faces several significant challenges and restraints that could impede its rapid expansion. One of the most persistent hurdles remains the delivery of RNAi molecules to target cells and tissues effectively and safely. The inherent instability of RNA molecules outside the cell and their susceptibility to degradation by nucleases necessitate sophisticated delivery vehicles, which can be costly to develop and manufacture. Achieving efficient cellular uptake and intracellular release of the active RNAi payload without causing toxicity or triggering an immune response remains a complex scientific and technical challenge. Consequently, the development of safe, efficient, and cost-effective delivery systems is paramount. Another significant restraint is the potential for off-target effects, where the RNAi molecule may unintentionally silence genes other than the intended target, leading to adverse events. While advances in RNAi design and screening have reduced this risk, it remains a critical consideration during drug development. The high cost of RNAi therapies is also a substantial barrier to widespread adoption. The complex manufacturing processes and the specialized nature of these therapeutics contribute to their elevated price points, which can limit patient access and place a considerable burden on healthcare systems. Furthermore, regulatory hurdles, while becoming more streamlined, can still be a lengthy and expensive process for novel RNAi therapeutics, requiring extensive preclinical and clinical data to demonstrate safety and efficacy. Finally, competition from other therapeutic modalities, such as gene editing technologies (e.g., CRISPR-Cas9) and next-generation gene therapies, presents an ongoing challenge, as these technologies also offer innovative approaches to treating genetic diseases. The market size for RNAi therapeutics is currently estimated to be over $3,200 million in the base year of 2025, but the pace of overcoming these challenges will dictate the trajectory of future growth.
The RNAi for therapeutic market is poised for dominance in several key regions and segments, driven by a confluence of factors including advanced research infrastructure, robust pharmaceutical pipelines, favorable regulatory environments, and significant patient populations.
Dominant Segments:
Type: siRNA (Small Interfering RNA): siRNA therapeutics are currently the most mature and widely adopted type of RNAi technology in the market. Their established efficacy and the ongoing success of marketed siRNA drugs have cemented their position. Companies like Alnylam Pharmaceuticals have significantly contributed to the growth of this segment. The robust pipeline of siRNA candidates targeting a wide array of diseases further solidifies its dominance, with projected market share exceeding 60% of the total RNAi therapeutics market by the forecast period. The ability of siRNA to precisely silence gene expression at the post-transcriptional level makes it highly attractive for a broad range of applications.
Application: Cardiovascular Diseases: The cardiovascular segment is emerging as a significant growth engine for RNAi therapeutics. The high prevalence of cardiovascular diseases globally, coupled with the identification of numerous genetic and molecular targets involved in their pathogenesis, presents a vast opportunity. RNAi offers novel mechanisms to address conditions like hypercholesterolemia, heart failure, and arrhythmias. The potential for lifelong treatment of these chronic conditions suggests a sustained and substantial revenue stream, with this segment expected to capture a market share of over 25% by the end of the forecast period.
Application: HBV (Hepatitis B Virus): The global burden of Hepatitis B Virus remains substantial, and RNAi offers a promising avenue for a functional cure by targeting the persistent viral DNA and its transcripts. The development of potent HBV-targeting RNAi therapies is a key focus for several companies, including Arbutus Biopharma. The potential for a curative therapy for HBV, which currently affects millions worldwide, positions this segment for significant market penetration, estimated to contribute over 15% to the RNAi therapeutics market.
Dominant Regions/Countries:
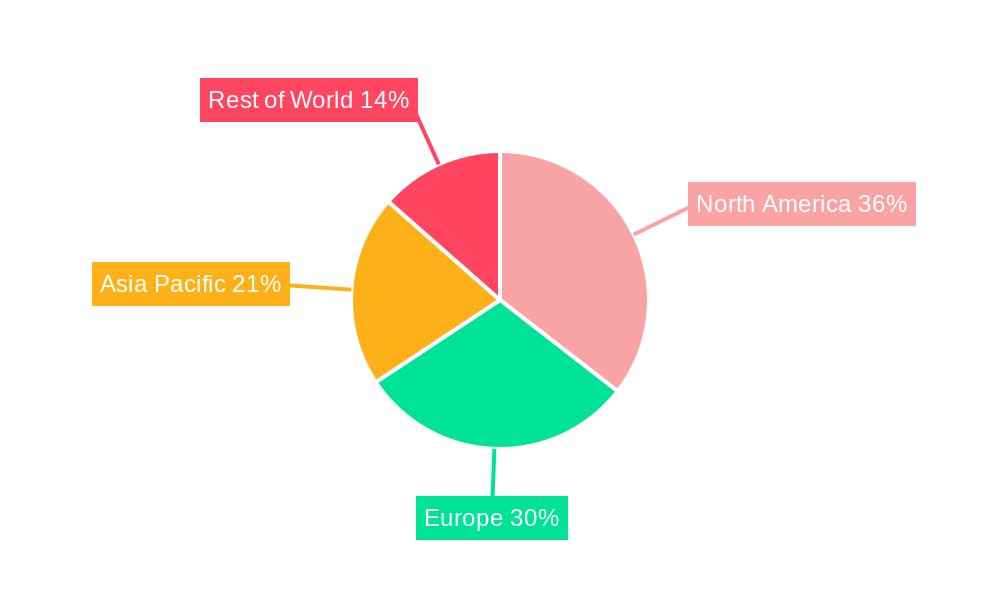
North America (United States): The United States stands as the leading region and country for the RNAi therapeutics market. This dominance is attributed to several factors:
Europe: Europe represents the second-largest market for RNAi therapeutics, characterized by strong governmental support for life sciences research and a growing emphasis on personalized medicine. Countries like Germany, the United Kingdom, and Switzerland are at the forefront of RNAi development and adoption. The presence of established pharmaceutical giants and a burgeoning biotech sector contribute to the region's significant market share.
The synergy between the dominant segments (siRNA, cardiovascular, HBV) and the leading regions (North America, Europe) creates a powerful market dynamic, where innovation in targeted therapies is met with robust demand and a supportive ecosystem for commercialization. The combined market value for these dominant segments is projected to represent over 90% of the global RNAi therapeutics market by the end of the forecast period.
The RNAi for therapeutic industry is experiencing a surge in growth catalyzed by several key factors. Ongoing advancements in delivery technologies, such as the refinement of lipid nanoparticles and the exploration of novel viral vectors and non-viral conjugates, are significantly enhancing the efficiency and safety of RNAi molecule delivery. This addresses a critical historical challenge, opening doors for a broader range of therapeutic targets and indications. Furthermore, the increasing success in clinical trials and the expanding portfolio of RNAi drugs gaining regulatory approval are building momentum and investor confidence, leading to increased R&D funding. The growing understanding of disease genetics and the identification of new, druggable targets further fuel the pipeline.
This report offers unparalleled comprehensive coverage of the RNAi for therapeutic market. It provides a granular analysis of market dynamics, including detailed segmentation by therapeutic type (siRNA, miRNA, shRNA) and application (Cancer, Cardiovascular, HBV, Others). The report meticulously outlines industry developments, tracks the progress of leading players, and identifies key growth catalysts. With a robust historical analysis from 2019-2024 and a forward-looking projection to 2033, based on a 2025 base and estimated year, this report equips stakeholders with the essential insights needed to navigate this rapidly evolving sector and capitalize on emerging opportunities. The estimated market size of over $7,500 million by 2033 underscores the immense potential covered within this analysis.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 41.2% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 41.2%.
Key companies in the market include Alnylam Pharmaceuticals, Arbutus Biopharma (Tekmira), Arrowhead, Dicerna Pharmaceuticals, Mirna Therapeutics, Quark Pharmaceuticals, RXi Pharmaceuticals, Silence Therapeutics, Benitec Biopharma, miRagen Therapeutics, Sylentis, Gradalis, Sirnaomics, Silenseed, .
The market segments include Type, Application.
The market size is estimated to be USD 181.1 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "RNAi for Therapeutic," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the RNAi for Therapeutic, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.