1. What is the projected Compound Annual Growth Rate (CAGR) of the Recombinant Human Osteopontin?
The projected CAGR is approximately 5%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Recombinant Human Osteopontin
Recombinant Human OsteopontinRecombinant Human Osteopontin by Type (>80%, >95%, >97%, Others, World Recombinant Human Osteopontin Production ), by Application (ELISA, WB, Antibody Production), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The global market for Recombinant Human Osteopontin (RHOP) is poised for significant expansion, projected to reach approximately $250 million by 2025 and continue its upward trajectory over the forecast period. This growth is fueled by increasing investments in biopharmaceutical research and development, particularly in areas like immunology, oncology, and regenerative medicine where osteopontin plays a crucial role. The compound's involvement in cellular signaling, immune modulation, and tissue repair makes it a valuable tool in drug discovery and development, driving demand for high-purity RHOP. Emerging applications in diagnostics, such as ELISA-based assays for disease biomarker detection, are also contributing to market dynamics. The market is characterized by a strong emphasis on product quality and purity, with segments like ">97%" purity demonstrating robust demand due to stringent research requirements. Key players in the market are focusing on innovation and expanding their product portfolios to cater to the evolving needs of research institutions and pharmaceutical companies worldwide.
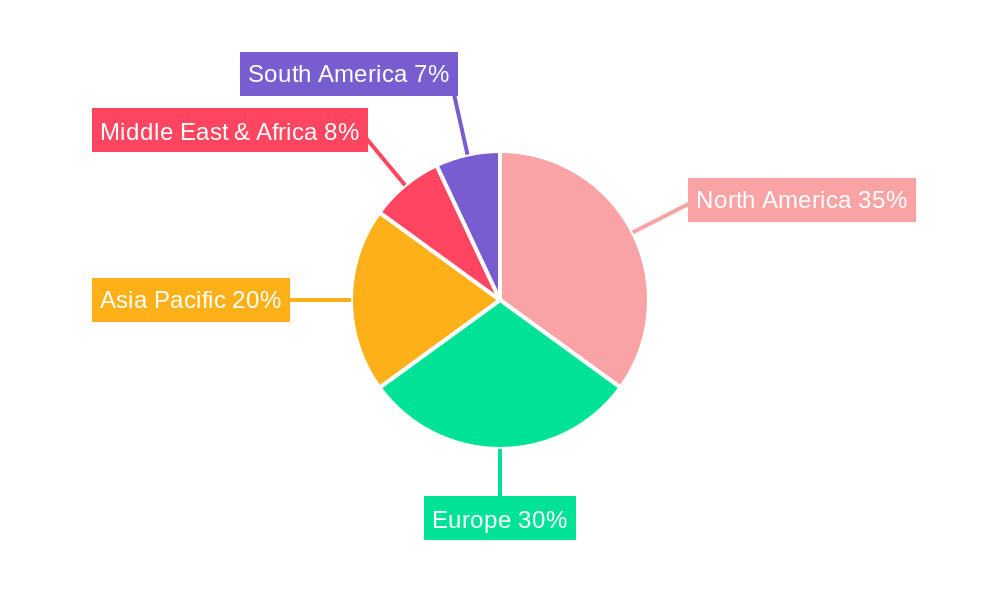
The market's expansion is further supported by advancements in recombinant protein production technologies, enabling more efficient and cost-effective manufacturing of RHOP. While growth is strong, certain restraints such as the high cost of production for highly pure recombinant proteins and potential regulatory hurdles in specific applications could temper the pace. However, the continuous exploration of osteopontin's therapeutic potential in treating chronic inflammatory diseases, autoimmune disorders, and cancer is expected to outweigh these challenges. Regionally, North America and Europe are anticipated to dominate the market, driven by their established biopharmaceutical research infrastructure and substantial R&D spending. The Asia Pacific region, particularly China and India, presents significant growth opportunities due to increasing investments in biotechnology and a growing pool of researchers exploring novel therapeutic targets. The competitive landscape is marked by the presence of several key companies, including ACROBiosystems, R&D Systems, and RayBiotech, who are actively engaged in strategic collaborations and product launches to capture market share.
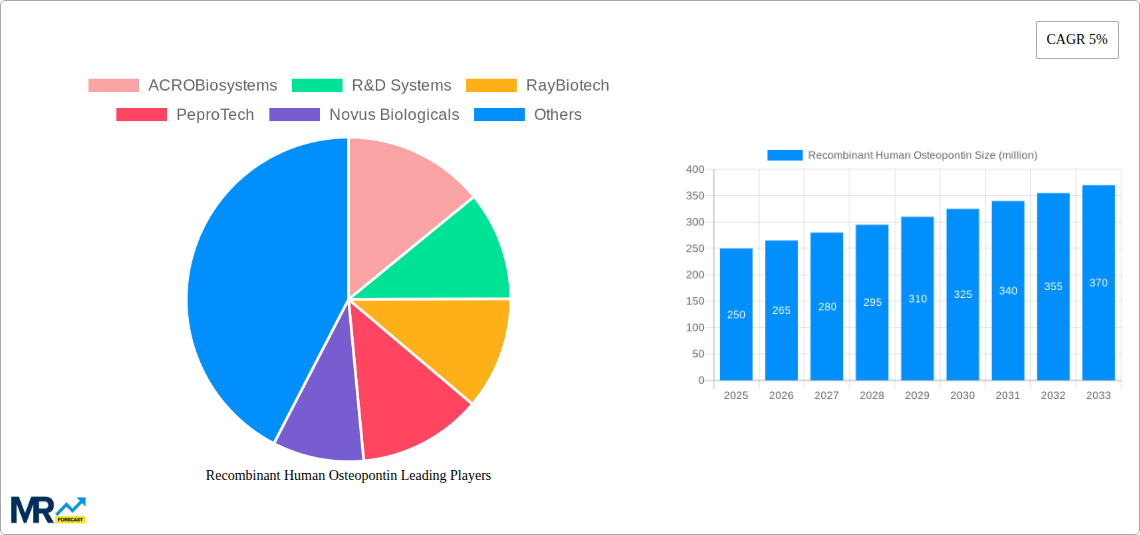
The global recombinant human osteopontin (RHOP) market is poised for substantial expansion, driven by an increasing understanding of its multifaceted biological roles and burgeoning applications across research and therapeutic development. Our comprehensive report, spanning the Study Period of 2019-2033 with a Base Year of 2025 and a Forecast Period from 2025-2033, illuminates critical trends shaping this dynamic sector. During the Historical Period of 2019-2024, the market witnessed steady growth, fueled by early-stage research into RHOP's involvement in immune modulation, bone remodeling, and cellular signaling. The Estimated Year of 2025 represents a pivotal point, with projections indicating a significant acceleration in market value, potentially reaching hundreds of millions of USD in the coming years. This growth is underpinned by advancements in recombinant protein expression technologies, leading to higher purity and yield, making RHOP more accessible for scientific inquiry. The market is segmented by purity levels, with >95% and >97% purity grades currently dominating due to their suitability for sensitive applications like ELISA and antibody production. The demand for these high-purity RHOP is projected to escalate as research delves deeper into the subtle functional differences that can be influenced by even minor impurities. Furthermore, the increasing prevalence of chronic inflammatory diseases, autoimmune disorders, and bone-related pathologies is directly translating into a heightened demand for RHOP as a research tool and a potential therapeutic agent. The development of novel diagnostic assays and targeted therapies leveraging RHOP's signaling pathways is a key trend. The market is also seeing a geographical shift, with North America and Europe currently leading in terms of consumption and production capabilities, while Asia-Pacific is emerging as a significant growth engine due to increasing R&D investments and the presence of contract research organizations. The interplay between academic research, pharmaceutical R&D, and the increasing commercialization of RHOP-based products is defining the trajectory of this market. Innovations in bioprocessing and purification techniques are continuously driving down production costs, thereby broadening the accessibility of RHOP to a wider research community and paving the way for its integration into more sophisticated applications. The overall sentiment is one of robust and sustained growth, driven by both fundamental scientific discovery and the pragmatic application of RHOP in addressing significant unmet medical needs. The potential for RHOP to play a crucial role in regenerative medicine and cancer immunotherapy further amplifies its market significance.
Several key factors are propelling the recombinant human osteopontin (RHOP) market forward during the Study Period of 2019-2033. Foremost among these is the deepening scientific understanding of osteopontin's (OPN) diverse biological functions. As research uncovers its intricate involvement in processes such as bone mineralization, immune cell trafficking, wound healing, angiogenesis, and tumorigenesis, the demand for high-quality RHOP as a research reagent intensifies. This increased comprehension fuels more sophisticated experiments, leading to the discovery of novel therapeutic targets and diagnostic markers. The Estimated Year of 2025 is expected to see a surge in such discoveries, further stimulating market growth. Secondly, the rising global burden of diseases where OPN plays a significant role, including osteoporosis, rheumatoid arthritis, inflammatory bowel disease, and various cancers, is a major driver. RHOP is indispensable for studying disease mechanisms, screening potential drug candidates, and developing preclinical models. The ability of RHOP to modulate immune responses also makes it a crucial tool in the development of immunotherapies and treatments for autoimmune disorders. Furthermore, advancements in biotechnology, particularly in recombinant protein expression and purification techniques, have led to the availability of highly pure and biologically active RHOP. This improved quality and accessibility, coupled with a potential for cost reduction as production scales up, are making RHOP more attractive for a broader range of research applications and even early-stage therapeutic development. The continuous innovation in biomanufacturing processes ensures a consistent supply of RHOP, meeting the escalating demands from research institutions and biopharmaceutical companies alike. The projected market value, potentially in the hundreds of millions of USD by 2025, is a testament to these powerful driving forces.
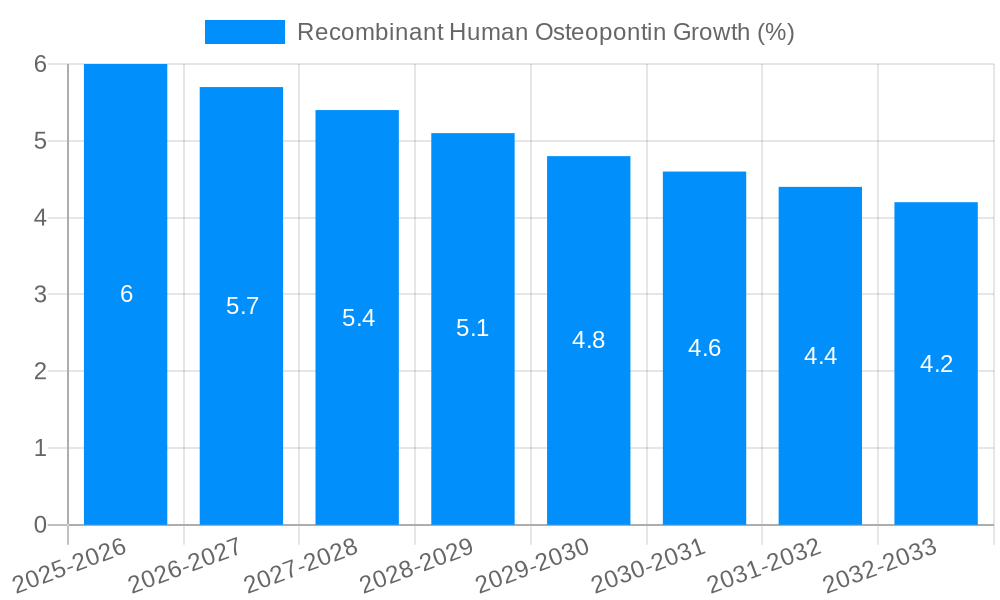
Despite the promising growth trajectory, the recombinant human osteopontin (RHOP) market faces several challenges and restraints that could temper its expansion between 2019 and 2033. A primary hurdle is the inherent complexity of OPN's biological functions. Its pleiotropic nature, meaning it can exert diverse effects depending on the cellular context and isoform, can make it challenging to fully elucidate its precise role in specific disease pathways. This complexity can slow down the translation of research findings into clear therapeutic applications, thereby limiting immediate market demand for certain applications. The Base Year of 2025 highlights this ongoing research phase. Another significant restraint is the cost associated with producing highly pure and consistently active RHOP. While advancements have been made, the manufacturing processes can still be expensive, particularly for large-scale production required for therapeutic development. This cost factor can limit the accessibility of RHOP for smaller research labs or early-stage companies. The competitive landscape, with numerous companies offering RHOP, can also lead to price pressures, potentially impacting profitability for manufacturers. Furthermore, regulatory hurdles associated with developing RHOP as a therapeutic agent are substantial. Extensive preclinical and clinical trials are required to demonstrate safety and efficacy, which are time-consuming and resource-intensive. The lengthy approval processes in major markets can be a significant deterrent. The potential for immune responses to recombinant proteins, even humanized ones, needs to be carefully managed and monitored, adding another layer of complexity to therapeutic development. Lastly, the reliance on funding for academic and governmental research can be a constraint, as budget allocations can fluctuate, impacting the demand for research reagents.
The global recombinant human osteopontin (RHOP) market is projected to see significant dominance from specific regions and segments during the Study Period of 2019-2033.
Dominant Regions:
North America (United States and Canada): This region is expected to maintain its leading position due to several compelling factors.
Europe (Germany, United Kingdom, France): Europe represents another substantial market, characterized by its strong scientific community and increasing R&D investments.
Dominant Segments:
Type: >95% and >97% Purity: These high-purity segments are projected to dominate the market, particularly in the Estimated Year of 2025 and beyond.
Application: ELISA and Antibody Production: These applications are expected to be major growth drivers.
The synergy between high-purity RHOP and its application in ELISA and antibody production, coupled with strong R&D investments and disease prevalence in regions like North America and Europe, will collectively dictate the dominant market share. The World Recombinant Human Osteopontin Production capacity is also increasing, driven by demand from these leading segments and regions, with companies like ACROBiosystems, R&D Systems, RayBiotech, and Sino Biological playing a crucial role in fulfilling this demand.
The recombinant human osteopontin (RHOP) industry is experiencing significant growth catalysts. A primary driver is the escalating scientific interest in osteopontin's (OPN) multifaceted roles in immune regulation, bone metabolism, and tumorigenesis. As research uncovers new therapeutic targets and diagnostic markers related to OPN, the demand for high-quality RHOP as a research tool intensifies. Furthermore, the increasing prevalence of chronic inflammatory and autoimmune diseases globally necessitates advanced research into their underlying mechanisms, with OPN being a key player. Advances in recombinant protein expression and purification technologies are also crucial, enabling the production of more affordable, pure, and biologically active RHOP, thus broadening its accessibility for various applications.
This report offers a comprehensive examination of the recombinant human osteopontin (RHOP) market, providing in-depth analysis from 2019 to 2033. It meticulously details market dynamics, including trends, driving forces, and challenges, with a focus on the Base Year of 2025 and projections through 2033. The report segments the market by product type (purity levels like >80%, >95%, >97%) and application (ELISA, WB, Antibody Production), highlighting key growth areas. It also identifies leading global players such as ACROBiosystems, R&D Systems, and RayBiotech, and outlines significant industry developments. The report's scope encompasses regional market analyses, with a particular emphasis on dominant regions and segments, offering valuable insights for stakeholders seeking to navigate and capitalize on the expanding RHOP market.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 5%.
Key companies in the market include ACROBiosystems, R&D Systems, RayBiotech, PeproTech, Novus Biologicals, Abcam, BioLegend, Sino Biological, Elabscience, FUJIFILM, Protein Alternatives, BBI Solutions, .
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Recombinant Human Osteopontin," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Recombinant Human Osteopontin, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.