1. What is the projected Compound Annual Growth Rate (CAGR) of the Orphan Drug Development?
The projected CAGR is approximately 10.94%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Orphan Drug Development
Orphan Drug DevelopmentOrphan Drug Development by Type (Pediatric Orphan Drugs, Adult Orphan Drugs), by Application (Congenital and Genetic Diseases, Tumors and Cancer, Endocrine Diseases, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The orphan drug market, dedicated to treatments for rare diseases, is poised for significant expansion. This growth is propelled by increased R&D investments, incentivized by regulatory exclusivity, and accelerated by advancements in genomics and personalized medicine. Growing patient and advocacy group awareness is also a key factor. The market is segmented by drug type and disease application, including congenital/genetic diseases, tumors and cancer, and endocrine disorders. North America leads, driven by high healthcare expenditure, with Asia-Pacific exhibiting substantial growth potential due to rising healthcare spending and rare disease awareness.
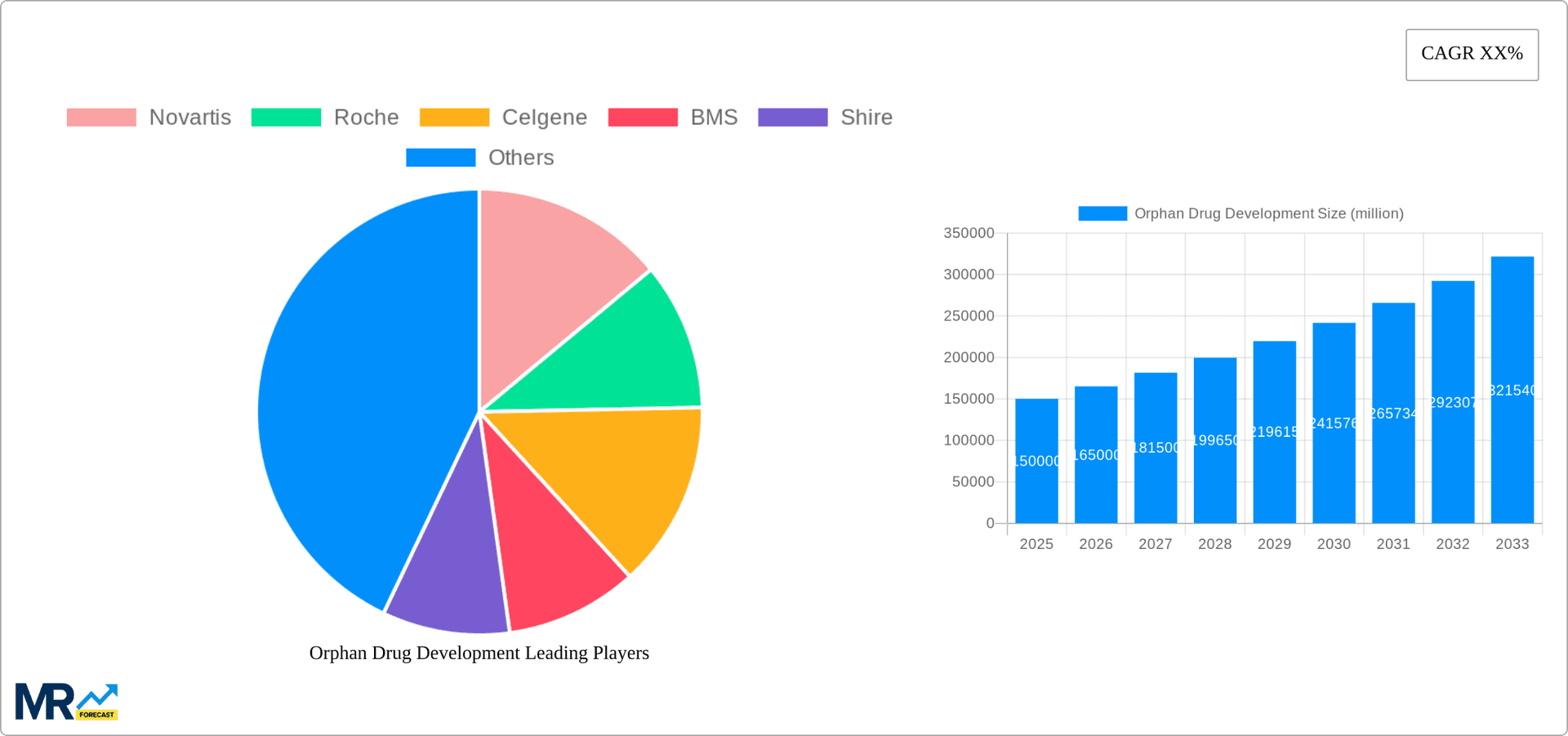

Despite opportunities, challenges persist, including high development costs and limited patient pools. Navigating complex regulatory pathways and ensuring equitable access to these vital medications remain critical issues. Sustained growth hinges on collaboration between pharmaceutical companies, regulatory bodies, patient groups, and healthcare providers. Continued innovation, strategic alliances, and supportive regulatory frameworks will be paramount. Key industry players such as Novartis, Roche, Bristol Myers Squibb, Takeda, Merck, Johnson & Johnson, Pfizer, Biogen, Sanofi, AstraZeneca, and Takeda are instrumental in this evolving market.
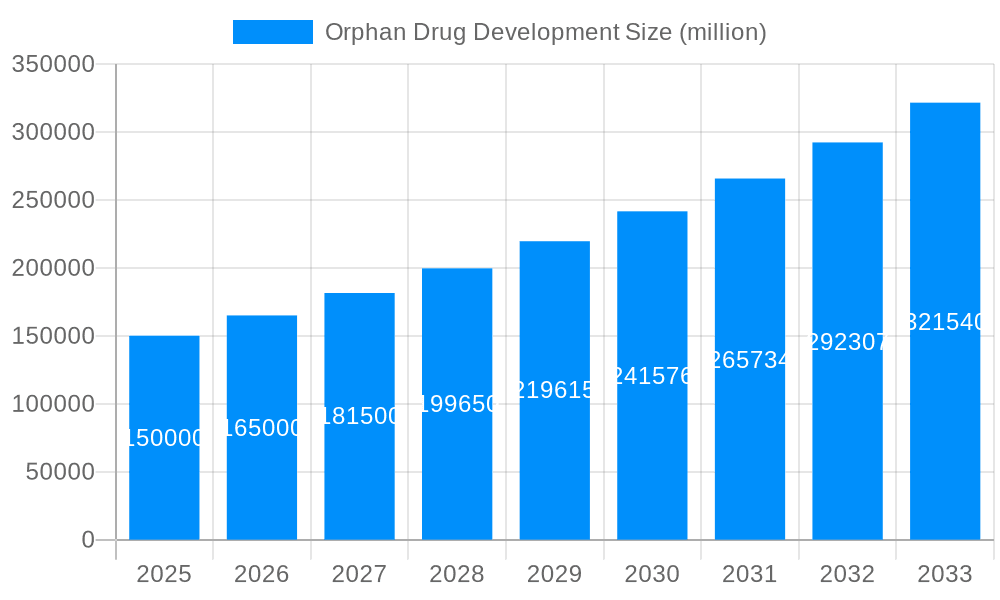

The orphan drug market, focused on treating rare diseases affecting fewer than 200,000 individuals in the US, is experiencing explosive growth. Driven by significant unmet medical needs, supportive regulatory frameworks like the Orphan Drug Act, and substantial financial incentives, the market is projected to reach multi-billion dollar valuations. The study period of 2019-2033 reveals a consistently upward trajectory. Our estimations for 2025 indicate a market value of $XXX million, with a projected value of $YYY million by 2033. This robust growth is not uniform across all segments; the specific drivers and challenges vary considerably depending on the type of orphan drug (pediatric vs. adult), the application (e.g., oncology vs. endocrine disorders), and geographical location. For instance, while oncology orphan drugs represent a significant portion of the market due to high pricing and substantial R&D investment, pediatric orphan drugs demonstrate slower growth due to the complexities of clinical trials in this vulnerable population. The historical period (2019-2024) saw a consolidation phase with major pharmaceutical players acquiring smaller biotech companies specializing in orphan drug development, leading to increased competition and accelerating innovation. Looking ahead to the forecast period (2025-2033), we anticipate continued market expansion driven by the pipeline of novel therapies and the rising awareness of rare diseases among healthcare professionals and patients. The base year of 2025 provides a crucial benchmark to assess the market’s progress and refine our future projections. Further analysis will delve into the specific drivers, challenges, and key players shaping the future of this dynamic market.
Several factors are accelerating orphan drug development. First, the Orphan Drug Act and similar international regulations provide substantial incentives, including tax breaks, expedited regulatory review processes, and market exclusivity periods, making the development of these drugs financially attractive. Second, there’s a growing awareness and advocacy for patients with rare diseases, leading to increased funding for research and clinical trials. Third, technological advancements in areas such as gene therapy and personalized medicine offer promising treatment options for previously incurable conditions, sparking intense R&D activity. Fourth, the high unmet medical needs within the rare disease community directly translates to high pricing strategies, making it financially viable for pharmaceutical companies to invest in this niche area. Finally, the success stories of previously marketed orphan drugs demonstrate the potential for significant returns on investment, thereby attracting further capital and talent into the sector. This positive feedback loop of regulatory support, technological advancements, and market incentives fuels the ongoing rapid expansion of the orphan drug development market.
Despite the substantial market potential, developing orphan drugs presents significant challenges. Patient recruitment for clinical trials is often difficult due to the low prevalence of rare diseases. This scarcity of patients increases the time and cost associated with clinical development and can significantly delay market entry. Furthermore, the inherent complexities of rare diseases, often involving diverse genetic mutations and unclear disease mechanisms, present substantial scientific hurdles. The high cost of drug development is also a major obstacle, often requiring substantial upfront investment with uncertain returns due to the relatively small patient population. Regulatory hurdles, although eased by supportive legislation, can still pose challenges, particularly in ensuring the long-term safety and efficacy of novel therapies. Finally, the commercialization of orphan drugs poses a unique challenge; reaching the limited and geographically dispersed patient population requires specialized distribution networks and engagement strategies.
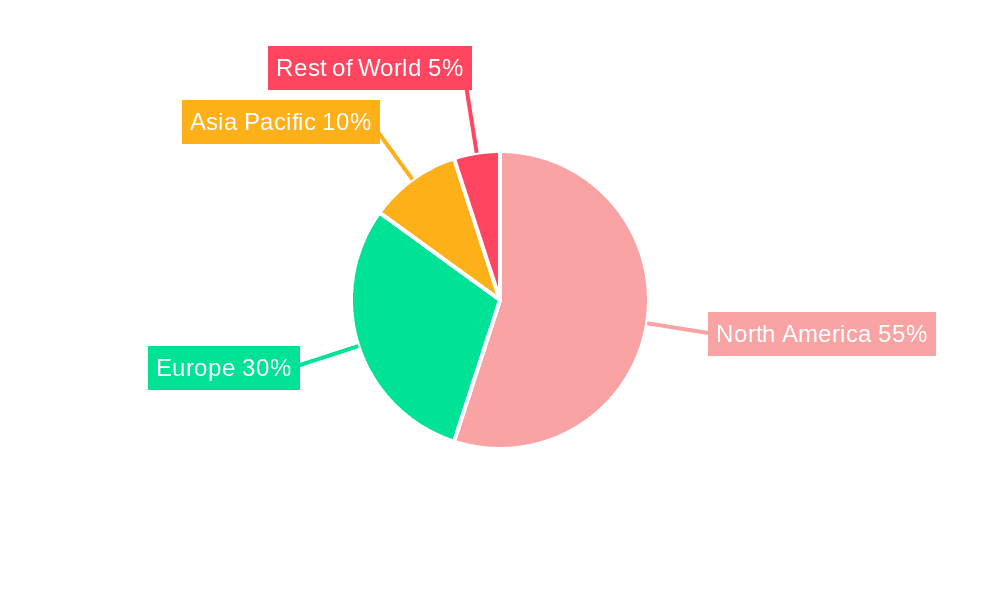
The North American market, particularly the United States, is expected to dominate the global orphan drug market throughout the forecast period. This dominance stems from several factors:
Within the segments, Adult Orphan Drugs focused on Tumors and Cancer are projected to be a major revenue driver.
Other segments, such as pediatric orphan drugs and those targeting endocrine diseases, will show considerable growth, though at a potentially slower pace due to complexities involved in clinical development and smaller patient populations.
The orphan drug industry benefits significantly from the converging forces of supportive government policies, technological breakthroughs in gene therapy and personalized medicine, and increased patient advocacy leading to greater awareness and funding. These elements accelerate R&D, enable development of highly effective drugs, and expand market access, all of which propel considerable growth.
This report provides a comprehensive analysis of the orphan drug development market, covering key trends, drivers, challenges, and future projections. It offers detailed segmentation by drug type, application, and geography, providing a granular understanding of this rapidly evolving market. The report further analyzes the key players and their strategic initiatives, offering valuable insights for stakeholders involved in the development, manufacturing, and commercialization of orphan drugs.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 10.94% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 10.94%.
Key companies in the market include Novartis, Roche, Celgene, BMS, Shire, Merck, J&J, Pfizer, Biogen, Sanofi, AstraZeneca, Takeda, .
The market segments include Type, Application.
The market size is estimated to be USD 236.27 billion as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in billion.
Yes, the market keyword associated with the report is "Orphan Drug Development," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Orphan Drug Development, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.