1. What is the projected Compound Annual Growth Rate (CAGR) of the Medical Device Outsourcing?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Medical Device Outsourcing
Medical Device OutsourcingMedical Device Outsourcing by Type (/> Finished Goods, Electronics, Raw Materials), by Application (/> Cardiology, Diagnostic Imaging, Orthopedic, IVD, Ophthalmic, General and Plastic Surgery, Drug Delivery), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2026-2034
The medical device outsourcing (MDO) market, valued at $142.16 billion in 2025, is experiencing robust growth driven by several key factors. Increasing demand for sophisticated medical devices, coupled with the rising prevalence of chronic diseases globally, fuels the need for efficient and cost-effective manufacturing solutions. Original Equipment Manufacturers (OEMs) are increasingly outsourcing manufacturing, assembly, and testing processes to specialized contract manufacturers (CMs) to leverage their expertise, advanced technologies, and global reach, enhancing scalability and reducing operational overhead. Furthermore, stringent regulatory requirements and the need for quality assurance are prompting OEMs to partner with experienced CMs possessing robust quality management systems. The diverse applications across cardiology, diagnostic imaging, orthopedics, and others, contribute to the market's expansive size.

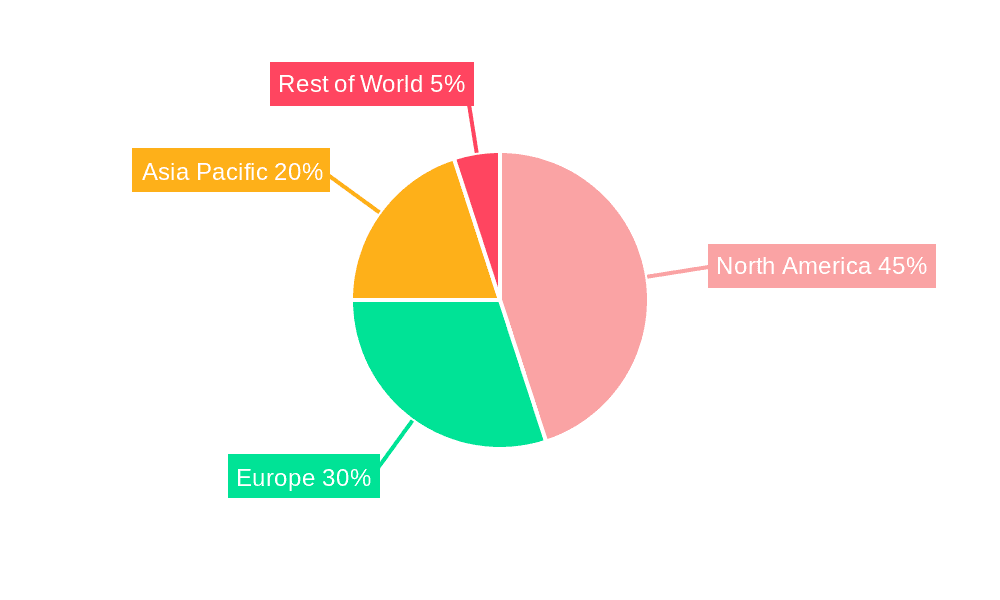
Significant regional variations exist within the MDO market. North America, particularly the United States, holds a dominant position due to its advanced healthcare infrastructure, presence of major OEMs, and a robust regulatory framework. However, Asia-Pacific, especially China and India, exhibits high growth potential, driven by expanding healthcare infrastructure investments and a growing middle class with increased disposable income. Europe also maintains a strong market share, with key players concentrated in Germany and the UK. Future growth will be shaped by technological advancements, such as the increasing adoption of 3D printing and automation in medical device manufacturing. Continued consolidation within the CM sector and the emergence of specialized service providers catering to niche areas within the medical device industry are also anticipated. While the exact CAGR is unavailable, considering market size and growth drivers, a conservative estimate would place annual growth in the range of 5-7% over the forecast period (2025-2033).
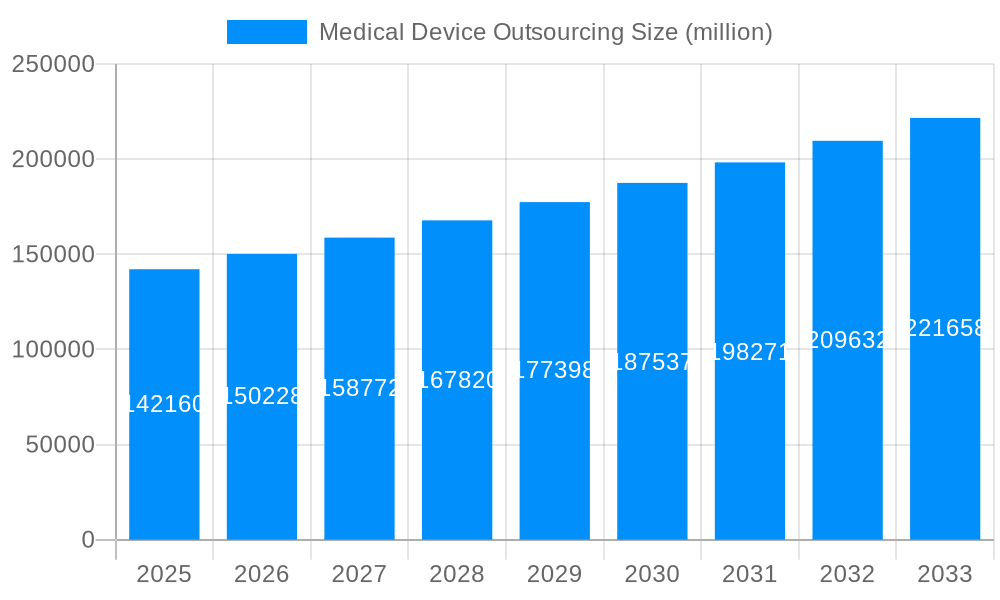

The medical device outsourcing market is experiencing robust growth, projected to reach several billion USD by 2033. This expansion is fueled by several key factors, including the increasing complexity of medical devices, the rising demand for cost-effective manufacturing solutions, and the growing focus on innovation within the healthcare industry. The market witnessed significant expansion during the historical period (2019-2024), with a Compound Annual Growth Rate (CAGR) exceeding expectations. This trend is expected to continue throughout the forecast period (2025-2033), driven by an increasing number of strategic partnerships between Original Equipment Manufacturers (OEMs) and Contract Manufacturers (CMs). The estimated market value in 2025 is already substantial, indicating a mature and competitive landscape. This report provides a comprehensive overview of the market, analyzing historical data (2019-2024), the present (base year 2025), and projecting future trends (2025-2033). Key market insights reveal a shift towards specialized outsourcing services, with OEMs increasingly focusing on core competencies like R&D and marketing while entrusting manufacturing and other operational aspects to specialized contract manufacturers. This trend is particularly prominent in segments like cardiology and diagnostic imaging, which demand high precision and regulatory compliance. The increasing adoption of advanced technologies such as automation, artificial intelligence, and 3D printing is further contributing to the growth of the market, enabling greater efficiency and precision in manufacturing processes. The market is also witnessing a rise in demand for flexible and agile outsourcing solutions that can adapt to changing market dynamics and evolving regulatory requirements. Finally, geographical expansion into emerging economies offering cost-effective manufacturing capabilities is another significant trend shaping the industry landscape.
Several key factors are driving the growth of the medical device outsourcing market. The increasing complexity of medical devices, particularly those incorporating advanced technologies like microelectronics and sophisticated software, necessitates specialized manufacturing capabilities beyond the resources of many OEMs. Outsourcing allows these companies to access cutting-edge technology, specialized expertise, and optimized manufacturing processes, leading to improved product quality and reduced development times. The stringent regulatory requirements within the medical device industry pose a significant challenge for OEMs. Outsourcing to contract manufacturers with established quality management systems (QMS) and experience navigating complex regulatory landscapes significantly reduces compliance risks and ensures products meet stringent safety and performance standards. Furthermore, cost optimization is a major driving force. Outsourcing manufacturing allows OEMs to reduce capital expenditures on infrastructure, equipment, and personnel, leading to improved profitability. The ability to leverage the economies of scale offered by large contract manufacturers further enhances cost-effectiveness. Finally, the growing demand for faster time-to-market necessitates efficient and flexible manufacturing solutions. Outsourcing enables OEMs to respond quickly to market demands and leverage the expertise and capacity of established contract manufacturers to accelerate product launches.
Despite its growth potential, the medical device outsourcing market faces several challenges. Maintaining intellectual property (IP) security is a major concern for OEMs. Effective contracts and robust IP protection mechanisms are critical to mitigate the risk of IP breaches. Ensuring consistent quality and compliance across multiple outsourcing partners can be challenging. Rigorous quality control processes, regular audits, and strong supplier relationships are essential to maintaining high product quality standards. Geographical limitations, such as varying regulatory environments and logistical complexities, can pose challenges for global outsourcing strategies. Careful consideration of these factors is crucial for successful global outsourcing initiatives. The management of supply chain disruptions, particularly evident during recent global events, is a significant risk. Diversifying supply chains, building resilient relationships with multiple suppliers, and developing contingency plans are vital strategies for mitigating this risk. Finally, the potential for communication barriers and cultural differences between OEMs and their outsourcing partners can impact project timelines and outcomes. Clear communication protocols, well-defined contracts, and close collaboration are vital to address these challenges and ensure successful partnerships.
The medical device outsourcing market is geographically diverse, with several regions exhibiting significant growth potential. North America, particularly the United States, remains a dominant market due to the presence of major OEMs and a robust regulatory framework. However, Asia-Pacific is emerging as a key growth region, driven by the increasing presence of medical device manufacturers and the expanding healthcare infrastructure. Europe also represents a significant market, with established regulatory bodies and a strong focus on innovation.
Finished Goods: This segment is poised for significant growth due to the increasing demand for complex medical devices requiring specialized manufacturing processes. The outsourcing of finished goods production allows OEMs to focus on product development and marketing, while leveraging the expertise and scale of contract manufacturers. This segment is projected to account for a substantial portion of the overall market value.
Electronics: The increasing integration of electronics in medical devices is driving substantial growth in the electronics segment. The demand for sophisticated miniaturized electronics, combined with the stringent quality and reliability requirements of the medical device industry, favors outsourcing to specialized contract manufacturers with expertise in this field. This segment is particularly critical for advanced diagnostic imaging and cardiology devices.
Cardiology: The cardiology segment is a major driver of growth due to the continuous innovation in cardiovascular devices and the rising prevalence of cardiovascular diseases. Outsourcing plays a crucial role in meeting the demand for sophisticated cardiac implants, pacemakers, and other critical devices. The high precision and regulatory demands of this segment make outsourcing a strategic necessity for many OEMs.
Diagnostic Imaging: The advancements in diagnostic imaging technology, particularly in areas like MRI, CT scans, and ultrasound, are driving demand for sophisticated electronics and high-precision manufacturing capabilities. Outsourcing is crucial for OEMs to effectively manage the complexity and regulatory requirements associated with these devices.
The paragraph above illustrates how these segments are dominating. The combination of geographic location and specific market segments creates multiple pockets of high growth. For example, the combination of North America and the Finished Goods segment, or Asia-Pacific and the Electronics segment, represents particularly strong growth opportunities.
Several factors are accelerating growth in the medical device outsourcing sector. Technological advancements like automation and 3D printing are boosting efficiency and enabling the production of more complex devices. The increasing prevalence of chronic diseases globally is driving demand for medical devices, further fueling the outsourcing market. Finally, the rising need for cost-effective manufacturing solutions is pushing OEMs to partner with contract manufacturers for greater cost efficiencies and improved time-to-market.
This report provides a detailed analysis of the medical device outsourcing market, covering historical data, current trends, and future projections. It identifies key market drivers, challenges, and growth opportunities, offering valuable insights for stakeholders across the medical device industry. The report includes in-depth segment analysis, geographical market breakdowns, and competitive landscape profiling of leading players, providing a comprehensive resource for decision-making.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of XX% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
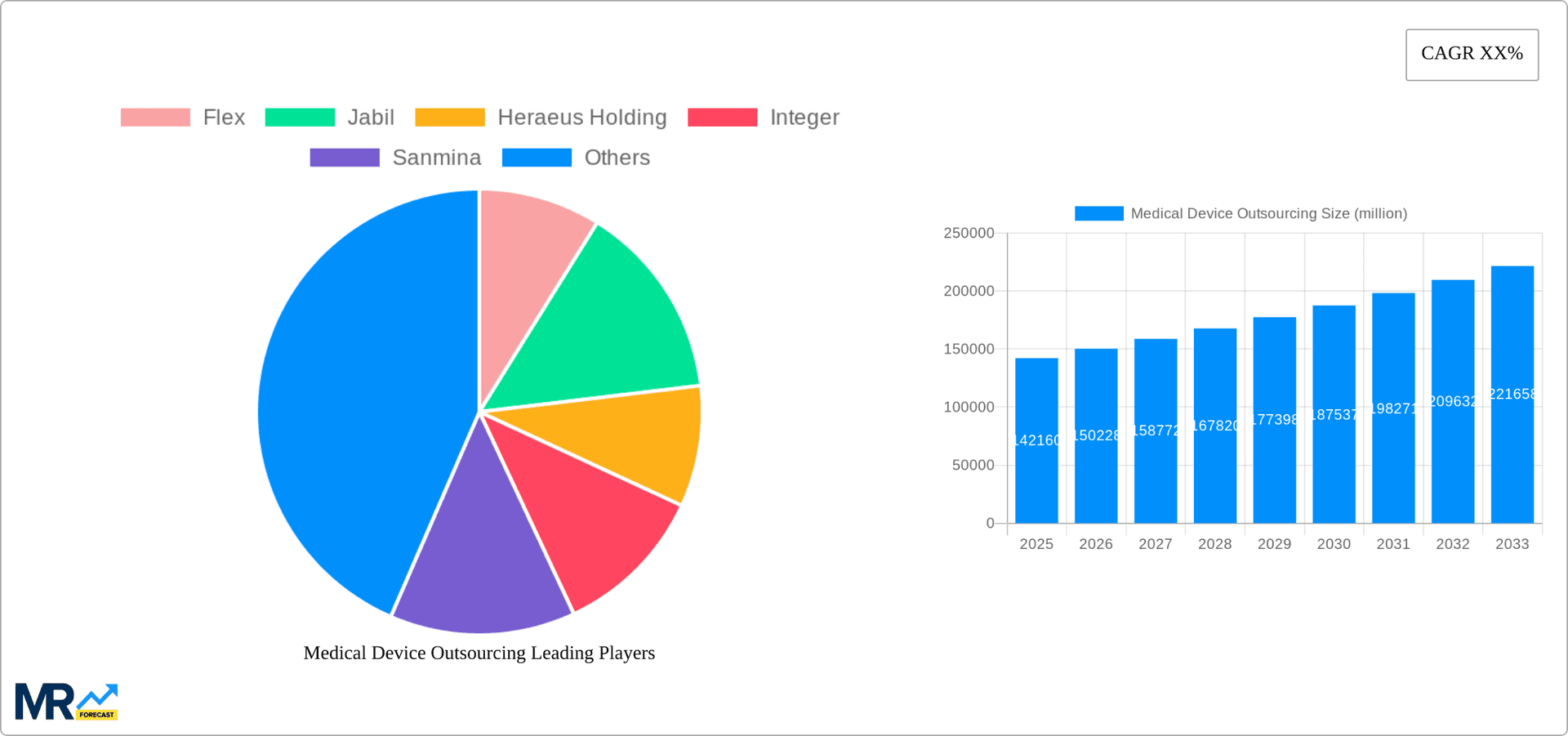
Key companies in the market include Flex, Jabil, Heraeus Holding, Integer, Sanmina, Plexus, TE Connectivity, Celestica, Tecomet, PPD, Cardinal Health, Eurofins Scientific, Intertek Group, SGS SA, Cadence Device, Seasky Medical, Scapa, Spectrum Solution, NAGL MedTech, Meridian Medical, MME group inc., Providence Enterprise USA, Inc., HDA Technology, Advantech Plastics, LLC, .
The market segments include Type, Application.
The market size is estimated to be USD 142160 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million.
Yes, the market keyword associated with the report is "Medical Device Outsourcing," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Medical Device Outsourcing, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.