1. What is the projected Compound Annual Growth Rate (CAGR) of the Medical Central Venous Elbow?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Medical Central Venous Elbow
Medical Central Venous ElbowMedical Central Venous Elbow by Application (Hospital, Clinic, World Medical Central Venous Elbow Production ), by Type (Single Cavity, Double Cavities, Three Cavities, World Medical Central Venous Elbow Production ), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
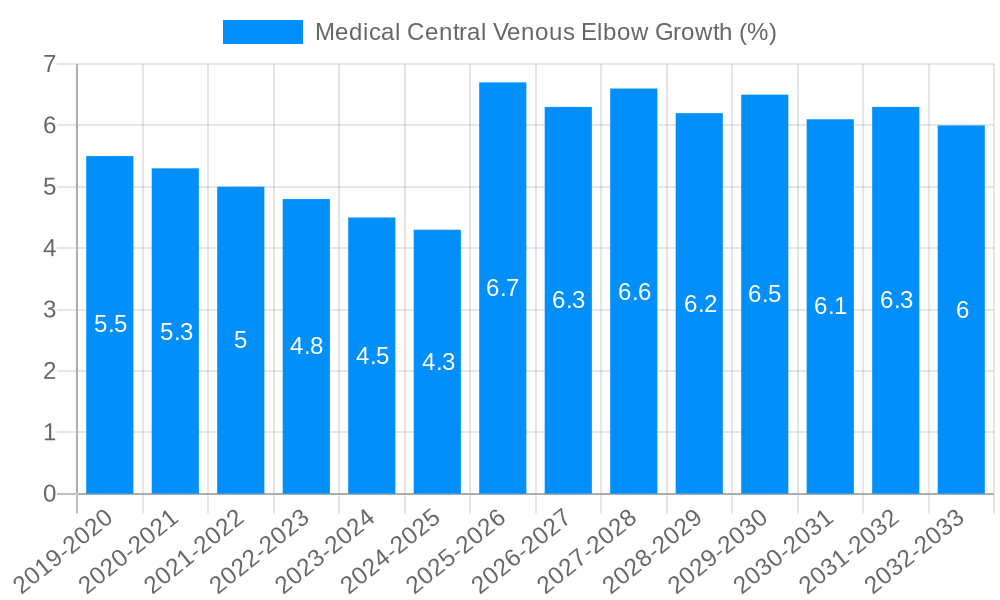
The global Medical Central Venous Elbow market is poised for robust expansion, projected to reach a substantial market size of approximately $650 million by 2025, with a compelling Compound Annual Growth Rate (CAGR) of around 7.5% expected throughout the forecast period (2025-2033). This significant growth is primarily fueled by an increasing prevalence of chronic diseases requiring long-term vascular access, such as cancer, kidney failure, and cardiovascular conditions. Advancements in medical technology, leading to the development of more sophisticated and patient-friendly central venous access devices, also act as a major driver. The rising demand for minimally invasive procedures further underpins market expansion, as these devices are integral to such interventions. Hospitals represent the largest application segment, driven by their central role in patient care and the high volume of procedures requiring central venous access. Within the type segmentation, double and triple cavity central venous elbows are expected to witness higher adoption due to their versatility in delivering multiple therapies simultaneously, addressing complex patient needs.
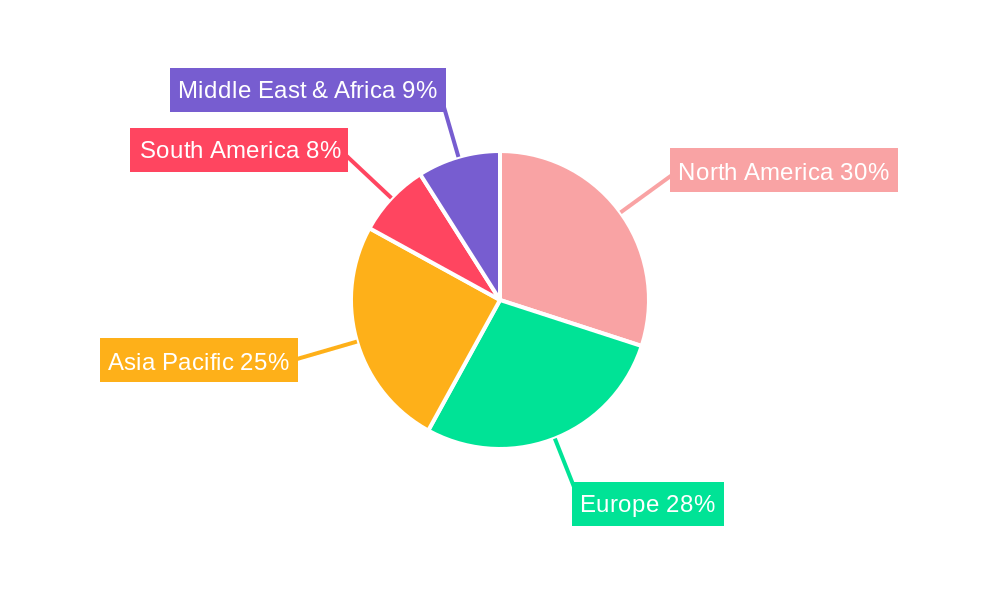
Geographically, North America and Europe currently lead the market, owing to well-established healthcare infrastructures, high healthcare expenditure, and a strong emphasis on adopting advanced medical devices. However, the Asia Pacific region is anticipated to exhibit the fastest growth rate, driven by a burgeoning healthcare sector, increasing disposable incomes, growing awareness of advanced medical treatments, and a large patient population. Emerging economies in this region are witnessing significant investments in healthcare infrastructure, further stimulating the demand for medical central venous elbows. Restraints to market growth include the high cost associated with advanced devices and stringent regulatory approvals, which can prolong market entry timelines. Nevertheless, the persistent need for effective long-term vascular access solutions and continuous innovation in product design are expected to outweigh these challenges, ensuring sustained market momentum.
This comprehensive report delves into the intricate dynamics of the Medical Central Venous Elbow market, meticulously analyzing trends, driving forces, challenges, and opportunities from 2019 to 2033, with a base and estimated year of 2025. The report aims to provide invaluable insights for stakeholders, leveraging a robust dataset and expert analysis to forecast future market trajectories. The World Medical Central Venous Elbow Production landscape is expected to witness substantial growth, driven by advancements in healthcare infrastructure and an increasing demand for minimally invasive procedures.
The report explores the nuances of World Medical Central Venous Elbow Production, anticipating a market valuation that will reach multi-million dollar figures by 2025 and continue its upward trajectory throughout the forecast period. Understanding the intricate supply chains, manufacturing processes, and regulatory landscapes is crucial, and this report offers an in-depth examination of these elements.
The Medical Central Venous Elbow market is currently experiencing a dynamic evolution, characterized by a confluence of technological advancements, shifting healthcare paradigms, and expanding applications. Over the study period of 2019-2033, particularly within the estimated year of 2025 and the subsequent forecast period of 2025-2033, a discernible trend towards enhanced product design and functionality is evident. Manufacturers are increasingly focusing on developing central venous catheters with improved biocompatibility, reduced thrombogenicity, and enhanced maneuverability, catering to the growing need for safer and more effective venous access solutions. The World Medical Central Venous Elbow Production is increasingly leaning towards single-use, sterile devices to mitigate the risk of healthcare-associated infections, a significant concern in hospital settings. Furthermore, there's a growing emphasis on user-friendliness for healthcare professionals, leading to innovations in insertion techniques and catheter materials. The historical period of 2019-2024 has laid the groundwork for this innovation, with initial investments in research and development paving the way for more sophisticated offerings. The market is witnessing a gradual shift towards specialized central venous catheters designed for specific patient populations and clinical scenarios, such as those requiring long-term venous access or experiencing particular medical conditions. This specialization not only improves patient outcomes but also drives niche market growth. The integration of advanced imaging guidance technologies during insertion procedures is also becoming more prevalent, further enhancing the safety and efficacy of central venous catheter placement. The increasing adoption of these advanced techniques is a significant trend that will continue to shape the market in the coming years. The demand for central venous catheters is also being influenced by the global rise in chronic diseases and the increasing complexity of patient care, necessitating reliable and long-term venous access solutions. This growing need for sustained venous access is a key driver for innovation and market expansion. The competitive landscape, as analyzed within this report, reveals a focus on product differentiation through features like antimicrobial coatings, pressure-activated safety valves, and radiopaque markers, all aimed at improving patient safety and procedural efficiency. The market's overall growth is projected to be robust, reflecting these ongoing positive trends.
Several potent forces are significantly propelling the growth and adoption of Medical Central Venous Elbow devices across the globe. A primary driver is the escalating prevalence of chronic diseases, including cancer, cardiovascular disorders, and end-stage renal disease. These conditions often necessitate prolonged intravenous therapies, including chemotherapy, parenteral nutrition, and hemodialysis, all of which rely heavily on secure and reliable central venous access. The increasing number of minimally invasive surgeries also contributes to this surge, as central venous catheters are integral to fluid management, drug administration, and hemodynamic monitoring during and after these procedures. Furthermore, the aging global population is a substantial demographic influence. As individuals age, their susceptibility to chronic ailments increases, thereby driving the demand for medical devices that facilitate long-term care and treatment. The continuous advancements in medical technology, particularly in the field of catheter design and material science, are also playing a crucial role. Innovations leading to improved biocompatibility, reduced infection rates, and enhanced patient comfort are directly contributing to market expansion. The World Medical Central Venous Elbow Production is also influenced by the increasing focus on patient safety and infection control within healthcare institutions. Manufacturers are responding by developing devices with features designed to minimize complications such as catheter-related bloodstream infections (CRBSIs) and mechanical phlebitis. The growing adoption of advanced diagnostic and therapeutic techniques, which often require precise and consistent venous access, further fuels this market. As healthcare systems worldwide prioritize evidence-based practices and optimal patient outcomes, the demand for high-quality, reliable central venous access devices is expected to remain strong, making it a consistently growing segment of the medical device industry. The expansion of healthcare infrastructure in emerging economies is also a significant catalyst, providing access to advanced medical treatments for a larger patient base.
Despite the promising growth trajectory, the Medical Central Venous Elbow market faces several significant challenges and restraints that could impede its full potential. A paramount concern is the inherent risk of complications associated with central venous catheterization, such as infection, thrombosis, and pneumothorax. These risks, even with advanced devices, necessitate stringent protocols and skilled insertion techniques, and any perceived increase in these complications can lead to heightened scrutiny and potential market hesitations. The substantial cost associated with some advanced central venous catheters, particularly those with specialized features or antimicrobial coatings, can be a significant barrier to adoption, especially in resource-limited healthcare settings. Reimbursement policies from government and private payers can also influence market dynamics; unfavorable reimbursement rates for certain types of catheters can restrict their widespread use. Stringent regulatory approval processes for medical devices, requiring extensive clinical trials and adherence to quality standards, can be time-consuming and costly for manufacturers, potentially delaying the introduction of new and innovative products to the market. Competition within the World Medical Central Venous Elbow Production landscape is fierce, with numerous established players and emerging companies vying for market share. This intense competition can lead to price pressures and necessitate continuous investment in research and development to stay ahead, impacting profit margins. The availability of alternative venous access methods, while not always a direct substitute, can also pose a competitive challenge in certain clinical scenarios. Furthermore, the need for continuous training and education for healthcare professionals on the latest insertion techniques and catheter management practices adds to the operational complexities for healthcare providers and can indirectly affect market penetration if such training is not readily accessible or effectively implemented. The evolving healthcare landscape, with shifts in treatment modalities and a growing emphasis on cost-effectiveness, also requires constant adaptation from device manufacturers.
The World Medical Central Venous Elbow Production market is poised for significant growth, with certain regions and segments demonstrating a clear dominance and substantial potential for further expansion.
Dominant Segments:
Application: Hospital:
Type: Double Cavities:
Dominant Regions/Countries:
North America (United States & Canada):
Europe (Germany, UK, France):
The Medical Central Venous Elbow industry is experiencing robust growth propelled by several key catalysts. The escalating global burden of chronic diseases, particularly cancer and cardiovascular ailments, necessitates prolonged and consistent venous access for treatment, directly fueling demand. Concurrently, the aging global population is a significant demographic driver, as elderly individuals are more prone to chronic conditions requiring such interventions. Advancements in medical technology, especially in catheter materials and design, are leading to safer, more effective, and user-friendly devices, encouraging wider adoption. The increasing preference for minimally invasive procedures, which often rely on central venous access for fluid management and drug delivery, also contributes to market expansion.
The Medical Central Venous Elbow market is populated by a number of key players who are instrumental in driving innovation and shaping the industry landscape. These companies are continuously involved in research, development, and manufacturing of advanced central venous access devices.
The Medical Central Venous Elbow sector has witnessed several noteworthy developments that have shaped its evolution and continue to influence market trends.
This comprehensive report offers an in-depth exploration of the Medical Central Venous Elbow market, providing a 360-degree view of its landscape. It meticulously analyzes market trends, key growth drivers, and prevailing challenges, offering insights into the forces shaping the World Medical Central Venous Elbow Production. The report also identifies dominant regions and segments, highlighting their strategic importance and future potential. Furthermore, it details significant industry developments and lists the leading players contributing to the market's advancement. This comprehensive analysis is crucial for stakeholders seeking to understand the current market dynamics and anticipate future opportunities within this vital segment of the medical device industry. The report's detailed segmentation by Application (Hospital, Clinic) and Type (Single Cavity, Double Cavities, Three Cavities) further enhances its utility for targeted market intelligence.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
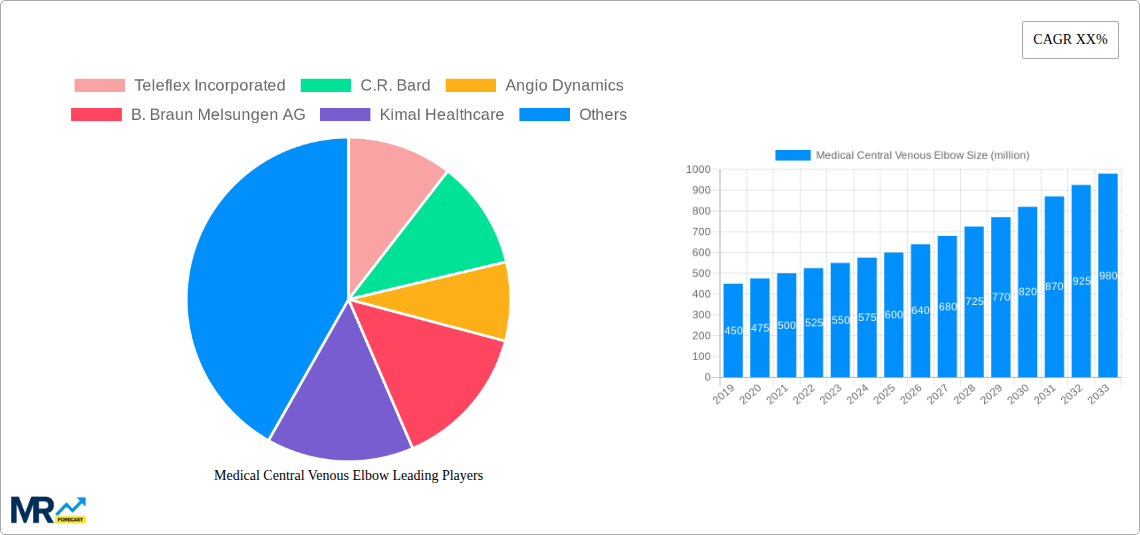
Key companies in the market include Teleflex Incorporated, C.R. Bard, Angio Dynamics, B. Braun Melsungen AG, Kimal Healthcare, Comed B.V., Medtronic Plc, Smiths Medical, Vygon (UK) Ltd, Becton, Dickinson and Company, Argon Medical Devices, Inc, Boston Scientific Corporation, Cook Medical Incorporated, Fresenius Kabi AG, Shenzhen Yixinda Medical New Technology Co., Ltd, Henan Johnson Minimally Invasive Medical Device Co., Ltd, Shandong Weixin Medical Device Co., Ltd, Shanghai Quanan Medical Device Co., Ltd, Shanghai Yixin Medical Equipment Co., Ltd, Shandong Baiduoan Medical Device Co., Ltd, Beijing Tiandihe Technology Co., Ltd, Guangdong Lily Medical Technology Co., Ltd, Shanghai Puyi Medical Device Co., Ltd, Jiangxi Sanxin Medical Technology Co., Ltd, Shandong Weigao Group Medical Polymer Products Co., Ltd, Nanjing Ningchuang Medical Equipment Co., Ltd, Jiangsu Ruijing Science and Technology Development Co., Ltd, Henan Shuguang Hui Zhikang Biotechnology Co., Ltd.
The market segments include Application, Type.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Medical Central Venous Elbow," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Medical Central Venous Elbow, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.