1. What is the projected Compound Annual Growth Rate (CAGR) of the Handheld Positive Expiratory Pressure Therapy Devices?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Handheld Positive Expiratory Pressure Therapy Devices
Handheld Positive Expiratory Pressure Therapy DevicesHandheld Positive Expiratory Pressure Therapy Devices by Type (Multi-gear, Single Gear), by Application (Hospital, Clinic, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
The global market for Handheld Positive Expiratory Pressure (PEP) Therapy Devices is poised for robust expansion, projected to reach an estimated USD 1,500 million by 2025, with a Compound Annual Growth Rate (CAGR) of approximately 8.5% from 2019 to 2033. This significant market size is fueled by a confluence of factors, primarily the increasing prevalence of respiratory diseases such as COPD, asthma, and cystic fibrosis, which necessitate effective airway clearance techniques. The growing aging population, a demographic highly susceptible to respiratory ailments, further bolsters demand. Moreover, advancements in device design, leading to more portable, user-friendly, and cost-effective handheld PEP devices, are actively driving market penetration. The rising awareness among healthcare professionals and patients regarding the benefits of PEP therapy, including mucus mobilization and improved lung function, is another critical growth driver. The shift towards home-based healthcare solutions and the continuous need for efficient management of chronic respiratory conditions also contribute to the market's positive trajectory.
The market is segmented by device type, with multi-gear devices anticipated to command a larger share due to their versatility in offering adjustable resistance levels, catering to a wider range of patient needs. Single-gear devices, however, will remain relevant for their simplicity and cost-effectiveness. Applications span across hospitals, clinics, and other healthcare settings, with hospitals likely representing the largest segment owing to their critical role in initial diagnosis and treatment of severe respiratory conditions. The market is characterized by intense competition among established players like Koninklijke Philips, PARI GmbH, and Monaghan Medical, alongside emerging innovators. Key market restraints include the potential for improper device usage leading to suboptimal outcomes and the availability of alternative treatment modalities. However, ongoing research and development focused on integrating smart features and enhancing therapeutic efficacy are expected to overcome these challenges, ensuring sustained growth and improved patient care in the handheld PEP therapy devices market.
Here's a unique report description for Handheld Positive Expiratory Pressure (PEP) Therapy Devices, incorporating your specified elements:
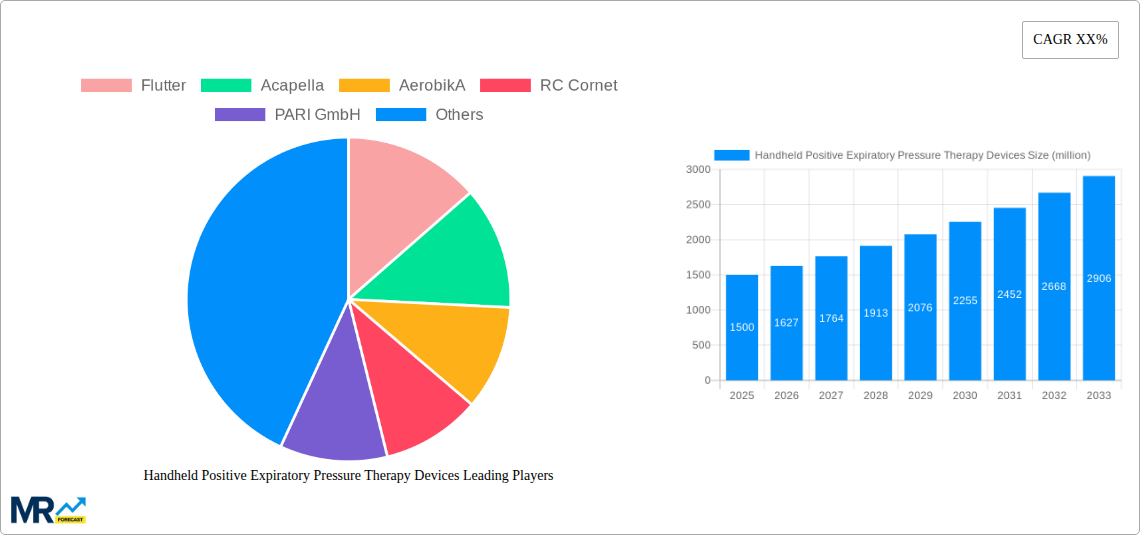
The global Handheld Positive Expiratory Pressure (PEP) Therapy Devices market is poised for significant expansion, projected to surge from an estimated 5.2 million units in the Base Year of 2025 to 8.9 million units by the end of the Forecast Period in 2033, showcasing a robust Compound Annual Growth Rate (CAGR) of 5.6%. This upward trajectory is underpinned by a confluence of factors, primarily the escalating prevalence of respiratory disorders such as Chronic Obstructive Pulmonary Disease (COPD), asthma, and cystic fibrosis, which necessitate effective airway clearance techniques. The increasing awareness among healthcare professionals and patients regarding the benefits of PEP therapy, including its non-invasive nature and ability to improve lung function and reduce exacerbations, is a critical driver. Furthermore, technological advancements leading to more user-friendly, portable, and affordable devices are democratizing access to this vital treatment. The historical period from 2019-2024 saw a steady adoption of PEP devices, with a growing understanding of their therapeutic value. The market's evolution is also being shaped by a shift towards home-based healthcare solutions, further boosting the demand for convenient, handheld PEP devices. The projected market size in the Estimated Year of 2025 stands at an impressive 5.5 million units, highlighting the immediate and substantial demand for these devices. Innovations in device design, focusing on enhanced patient compliance through ergonomic features and adjustable resistance levels, are also contributing to the positive market sentiment. The study period, spanning from 2019 to 2033, encompasses both the foundational growth phases and the anticipated acceleration in market penetration. The inherent simplicity and efficacy of handheld PEP devices, compared to more complex mechanical ventilators or chest physiotherapy, position them as a cornerstone in the management of chronic respiratory conditions for millions worldwide.
The robust growth witnessed and anticipated in the Handheld Positive Expiratory Pressure (PEP) Therapy Devices market is primarily propelled by the relentless surge in the global burden of respiratory diseases. Conditions like COPD, asthma, and cystic fibrosis, characterized by mucus buildup and airway obstruction, are becoming increasingly prevalent, especially in aging populations and regions with higher pollution levels. This escalating disease burden directly translates into a greater need for effective and accessible airway clearance solutions, with handheld PEP devices emerging as a preferred choice due to their portability and ease of use. Beyond the sheer volume of patients, a significant driving force is the growing clinical evidence substantiating the efficacy of PEP therapy. Numerous studies have demonstrated its ability to mobilize secretions, improve lung function, reduce dyspnea, and decrease the frequency and severity of respiratory exacerbations. This evidence base empowers healthcare providers to confidently recommend and prescribe PEP devices. Moreover, the expanding healthcare infrastructure and increasing disposable incomes in emerging economies are opening up new markets and driving demand for these therapeutic tools. Patients are becoming more proactive in managing their health, seeking out convenient and effective self-management strategies, which PEP devices readily offer. The shift towards patient-centric care models also favors the adoption of devices that empower individuals to manage their condition at home, thereby reducing reliance on healthcare facilities and enhancing their quality of life.
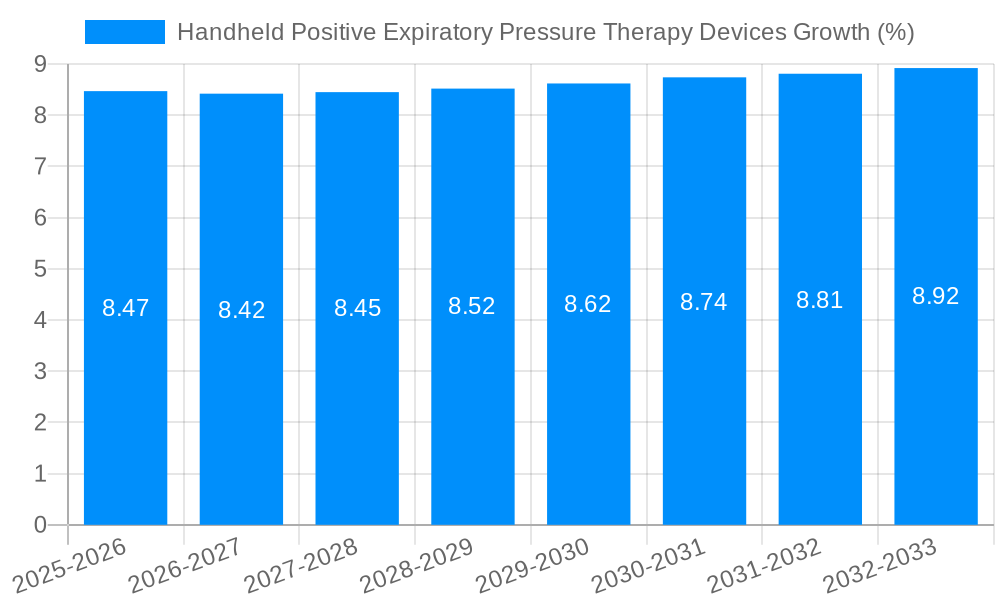
Despite the promising growth trajectory, the Handheld Positive Expiratory Pressure (PEP) Therapy Devices market faces several inherent challenges and restraints that could temper its expansion. A significant hurdle remains the issue of patient compliance. While PEP therapy is effective, consistent and correct usage is paramount for achieving optimal therapeutic outcomes. Lack of proper patient education, forgetfulness, or the perceived inconvenience of regular use can lead to suboptimal adherence, diminishing the overall effectiveness of the treatment. This is further compounded by the varying levels of health literacy across different demographics and geographical regions. Another critical restraint is the reimbursement landscape, which can be complex and inconsistent. In some regions, adequate insurance coverage for PEP devices may be lacking or limited, creating a financial barrier for a substantial portion of the patient population, particularly those with limited financial resources. This can disproportionately affect the adoption rates in developing economies. Furthermore, while the market is expanding, the presence of counterfeit and substandard products can also pose a threat. These imitation devices may not deliver the required therapeutic pressure, potentially leading to ineffective treatment or even adverse effects, thereby eroding patient and clinician trust in the technology. Lastly, the ongoing development and refinement of alternative airway clearance techniques and technologies, such as oscillating PEP devices or advanced manual chest physiotherapy methods, could present a competitive challenge, though handheld PEP devices generally offer a more accessible and cost-effective solution.
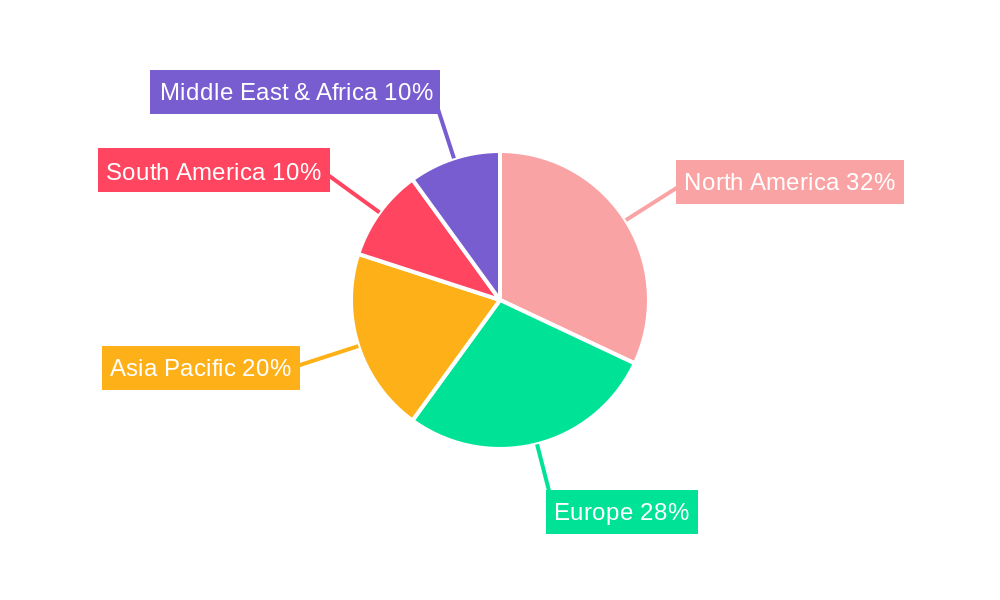
The Handheld Positive Expiratory Pressure (PEP) Therapy Devices market is anticipated to witness a significant dominance in terms of market share and growth within North America, driven by a confluence of favorable factors. The region boasts a highly developed healthcare infrastructure, with a strong emphasis on respiratory care and advanced treatment modalities. The presence of leading healthcare providers and research institutions fosters continuous innovation and early adoption of new medical devices.
North America's dominance is further bolstered by a high prevalence of respiratory diseases such as COPD and asthma, fueled by lifestyle factors and environmental conditions. The region also exhibits a higher disposable income, enabling greater patient affordability and accessibility to advanced healthcare solutions. Robust reimbursement policies and a well-established insurance framework for medical devices contribute significantly to market penetration. The study period (2019-2033) will see North America consistently leading in terms of unit sales and revenue generated from handheld PEP devices. The Estimated Year of 2025 projects North America to account for a substantial portion of the global market share, with units sold likely exceeding 2.1 million units.
Within the Type segment, Multi-gear PEP devices are projected to dominate. These devices offer adjustable resistance levels, allowing for personalized therapy tailored to individual patient needs and progression. This adaptability is crucial for managing a wide spectrum of respiratory conditions and improving patient compliance. The ability to progressively increase resistance as a patient's lung function improves makes multi-gear devices a more versatile and long-term solution. This segment is expected to represent approximately 65% of the total market units by the end of the forecast period. The historical data from 2019-2024 indicates a strong preference for these adjustable devices, and this trend is expected to continue and amplify. The Base Year of 2025 will see multi-gear devices selling an estimated 3.6 million units globally, with North America being the primary consumer.
In terms of Application, the Hospital segment is expected to maintain a leading position, particularly in the initial stages of diagnosis and treatment initiation. Hospitals are the primary point of care for many patients diagnosed with severe respiratory conditions, and PEP therapy is often prescribed and initiated under medical supervision within these settings. The availability of trained healthcare professionals ensures proper device usage and monitoring. Furthermore, hospital procurement of medical equipment often involves bulk purchases, contributing to higher unit sales in this segment. While home-based care is growing, the initial prescription and management often originate in hospitals. The Estimated Year of 2025 sees hospital applications accounting for approximately 45% of the total market units. However, the "Others" segment, which encompasses home healthcare settings and individual patient purchases, is poised for significant growth, driven by the trend towards remote patient management and the convenience of self-administered therapy. By 2033, the gap between hospital and home-based applications is expected to narrow considerably.
The Handheld Positive Expiratory Pressure (PEP) Therapy Devices industry is fueled by several powerful growth catalysts. The escalating global prevalence of chronic respiratory diseases like COPD and asthma remains a primary driver, creating an ever-expanding patient pool requiring effective airway clearance solutions. Furthermore, increasing patient awareness and demand for self-management tools empower individuals to actively participate in their treatment, thereby boosting the adoption of convenient devices like handheld PEP. Technological advancements leading to more user-friendly, portable, and affordable devices also play a crucial role in broadening market access.
This comprehensive report delves deep into the Handheld Positive Expiratory Pressure (PEP) Therapy Devices market, offering an exhaustive analysis of its growth dynamics. It meticulously examines the study period from 2019-2033, providing critical insights into the Base Year (2025) and Estimated Year (2025) market valuations and future projections. The report scrutinizes the driving forces behind market expansion, including the rising incidence of respiratory ailments and advancements in product design. It also critically evaluates the challenges and restraints, such as patient compliance issues and reimbursement complexities. A significant focus is placed on identifying key regions and dominant market segments, including the types (Multi-gear, Single Gear) and applications (Hospital, Clinic, Others) that are shaping the market landscape. The report further highlights crucial industry developments and identifies the leading players actively contributing to the market's evolution.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Flutter, Acapella, AerobikA, RC Cornet, PARI GmbH, TurboForte, Bottle PEP, USL Medical, Aptalis Pharma, Koninklijke Philips, Monaghan Medical, WyMedical, .
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Handheld Positive Expiratory Pressure Therapy Devices," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Handheld Positive Expiratory Pressure Therapy Devices, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.