1. What is the projected Compound Annual Growth Rate (CAGR) of the Germany Hepatitis C Testing Market?
The projected CAGR is approximately 21.2%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Germany Hepatitis C Testing Market
Germany Hepatitis C Testing MarketGermany Hepatitis C Testing Market by Test Types (HCV Serologic Tests (HCV Ab), by Technology Type (ELISA, PCR), by End User (Hospitals, Diagnostics Labs, Others), by Europe (Germany, France, Italy, United Kingdom, Netherlands, Rest of Europe) Forecast 2026-2034
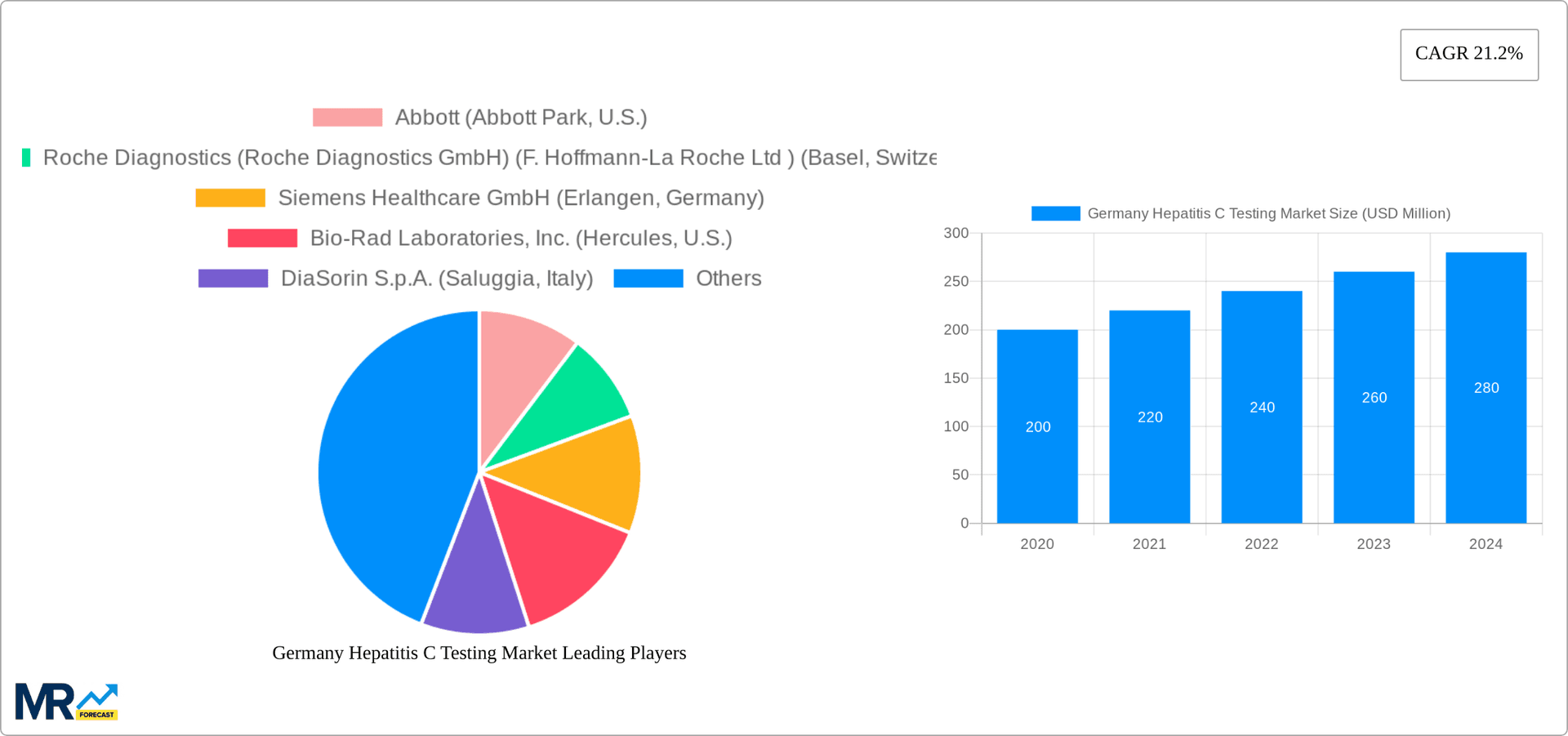
The Germany Hepatitis C Testing Market is thriving, with a valuation of 106.8 USD Million and a robust CAGR of 21.2%. This growth is attributed to several factors: the surge in Hepatitis C prevalence, the growing awareness of the disease and its testing, the development of advanced and cost-effective testing technologies, increasing government initiatives for disease screening and prevention, and the expanding healthcare infrastructure. The market's growth is further fueled by the entry of numerous domestic and international players, leading to intensified competition and innovation.

Increasing Disease Awareness, Early Detection, and Diagnosis Hepatitis C is a severe liver disease caused by the hepatitis C virus (HCV). With no vaccine available, early detection and diagnosis are crucial for effective treatment and management. Germany has a comprehensive healthcare system that promotes regular health checkups and screening for various infectious diseases, including Hepatitis C. Collaboration between healthcare providers, community organizations, and government agencies raises public awareness about viral hepatitis, its transmission, prevention, and management.
Government Initiatives to Combat Hepatitis C The German government has been at the forefront of implementing comprehensive strategies to combat viral hepatitis, emphasizing prevention, surveillance, early detection, and access to testing and treatment. The Federal Ministry of Health has established guidelines and programs to raise awareness, promote vaccination, and enhance the accessibility of affordable testing and treatment for Hepatitis C.

Advancements in Diagnostic Technologies: A Paradigm Shift in Detection and Management The Germany Hepatitis C Testing Market is experiencing a significant transformation driven by continuous technological breakthroughs in diagnostic methodologies. The market is witnessing a pronounced shift from older, less sensitive serological assays to more definitive and precise molecular testing. The increasing adoption of highly sensitive and specific Nucleic Acid Amplification Tests (NATs), such as Polymerase Chain Reaction (PCR) and Reverse Transcription Polymerase Chain Reaction (RT-PCR), is revolutionizing the field. These advanced NATs allow for the accurate and rapid detection and precise quantification of Hepatitis C Virus (HCV) RNA, leading to earlier and more reliable diagnoses. This enhanced accuracy and speed in diagnosis are directly contributing to improved patient outcomes and a more proactive approach to Hepatitis C management.
Rising Number of Risk Factors and Heightened Awareness: Fueling Testing Demand The prevalence of Hepatitis C is intrinsically linked to various risk factors that contribute to its transmission. Key among these are intravenous drug use, engagement in unprotected sexual practices with an infected individual, occupational exposure to contaminated blood, and healthcare-associated infections (nosocomial infections). The ongoing presence and, in some instances, the increasing incidence of these risk factors directly influence the demand for Hepatitis C testing within Germany. As the population becomes more informed about potential health risks associated with these behaviors and conditions, and as a greater emphasis is placed on individual health and well-being, the demand for comprehensive Hepatitis C testing is expected to rise, serving as a significant driver for market expansion.
Cost and Reimbursement Barriers The high cost of Hepatitis C testing can pose a challenge to its widespread accessibility. Diagnostic tests, particularly molecular assays, require specialized equipment and trained personnel, contributing to their higher expenses. Limited reimbursement for testing can also hinder individuals' access to screening and early diagnosis, potentially hindering the market's growth.
Key Region: Western Europe Western Europe, including Germany, accounts for a significant share of the global Hepatitis C Testing Market and is projected to maintain dominance in the coming years. The region has a high prevalence of Hepatitis C, advanced healthcare infrastructure, robust government initiatives for disease control, and universal access to healthcare services.
Dominant Segment: HCV Serologic Tests HCV Serologic Tests, particularly HCV antibody tests (HCV Ab), constitute the largest segment in the Germany Hepatitis C Testing Market. These tests are widely used for initial screening due to their high sensitivity and cost-effectiveness. The widespread availability and adoption of HCV Ab tests in hospitals, diagnostics labs, and community health centers contribute to their market dominance.
Technological Advancements: Towards Accessible and Efficient Diagnostics The relentless pace of technological innovation in the diagnostic sector is a primary growth catalyst for the Germany Hepatitis C Testing Industry. The development and increasing availability of non-invasive testing devices, particularly those designed for point-of-care (POC) applications, are poised to significantly boost market growth. These cutting-edge POC devices offer the distinct advantage of providing rapid, accurate, and remarkably convenient testing options. This accessibility is especially critical for enabling early detection and facilitating timely management of Hepatitis C, particularly in diverse healthcare settings, including those with limited resources or widespread geographical reach.
Increasing Public-Private Collaborations: A Unified Front Against Hepatitis C A crucial element driving the progress within the Germany Hepatitis C Testing Industry is the growing synergy and collaboration between public and private entities. Strategic partnerships involving government health agencies, dedicated healthcare providers, and innovative biotechnology companies are instrumental in the collective fight against Hepatitis C. These collaborations foster a fertile environment for robust research and development, leading to the creation of novel and improved testing technologies. Furthermore, they play a pivotal role in strengthening healthcare infrastructure, expanding equitable access to both diagnostic tools and life-saving treatments, and in the effective implementation of comprehensive prevention and control strategies. This concerted effort amplifies the impact of individual initiatives and accelerates the journey towards Hepatitis C elimination.
This comprehensive report provides an in-depth analysis of the Germany Hepatitis C Testing Market, including market size, trends, dynamics, drivers, restraints, competitive landscape, and future prospects. The report offers valuable insights into the market's key segments, emerging technologies, and regulatory frameworks.
Drivers: Rising Hepatitis C prevalence, increased awareness, advanced testing technologies, government initiatives, expanding healthcare infrastructure.
Restraints: Cost and reimbursement barriers, limited accessibility to testing.
Opportunities: Technological advancements in non-invasive testing, increasing public-private collaborations.
Challenges: Ensuring equitable access to diagnostics, addressing the stigma associated with Hepatitis C.
The Germany Hepatitis C Testing Market exhibits a competitive pricing landscape. The cost of testing varies depending on the type of test performed, testing facility, and geographical location. The availability of reimbursement schemes and government funding can influence the pricing of testing services.
Germany plays a significant role in the global import and export of Hepatitis C testing kits and reagents. The country imports testing components and equipment from various international suppliers, while it also exports its own testing products to other countries. The import and export dynamics are influenced by factors such as market demand, technological advancements, and international trade regulations.
The Germany Hepatitis C Testing Market is characterized by a growing number of patents and trademarks related to diagnostic technologies, test kits, and related products. Companies invest heavily in research and development to gain intellectual property protection for their innovations. Patent and trademark analysis provides insights into the competitive landscape and the potential for market growth.

| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 21.2% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 21.2%.
Key companies in the market include Abbott (Abbott Park, U.S.), Roche Diagnostics (Roche Diagnostics GmbH) (F. Hoffmann-La Roche Ltd ) (Basel, Switzerland), Siemens Healthcare GmbH (Erlangen, Germany), Bio-Rad Laboratories, Inc. (Hercules, U.S.), DiaSorin S.p.A. (Saluggia, Italy), Other Prominent Players, Abbott (Abbott Park, U.S.), Roche Diagnostics (Roche Diagnostics GmbH) (F. Hoffmann-La Roche Ltd ) (Basel, Switzerland), Siemens Healthcare GmbH (Erlangen, Germany), Bio-Rad Laboratories, Inc. (Hercules, U.S.), DiaSorin S.p.A. (Saluggia, Italy), Other Prominent Players.
The market segments include Test Types, Technology Type, End User.
The market size is estimated to be USD 106.8 USD Million as of 2022.
Increasing Public Awareness for Safer Medicines to Stimulate Market Value.
Manufacturers focusing on the Development of Mitral Valve Product will drive the Market.
Absence of Nationwide HCV Testing Program in Germany to Limit Growth.
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 2850, USD 3850, and USD 4850 respectively.
The market size is provided in terms of value, measured in USD Million and volume, measured in million units.
Yes, the market keyword associated with the report is "Germany Hepatitis C Testing Market," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Germany Hepatitis C Testing Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.