1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Phase IV Clinical Trials Market?
The projected CAGR is approximately 5.1%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Europe Phase IV Clinical Trials Market
Europe Phase IV Clinical Trials MarketEurope Phase IV Clinical Trials Market by Deployment (In-house, Outsource), by Disease Indication (Oncology, Neurology, Cardiology, Infectious Disease, Metabolic Disorder, Renal/Nephrology, Others), by Type (Interventional, Non-interventional), by Forecast 2026-2034
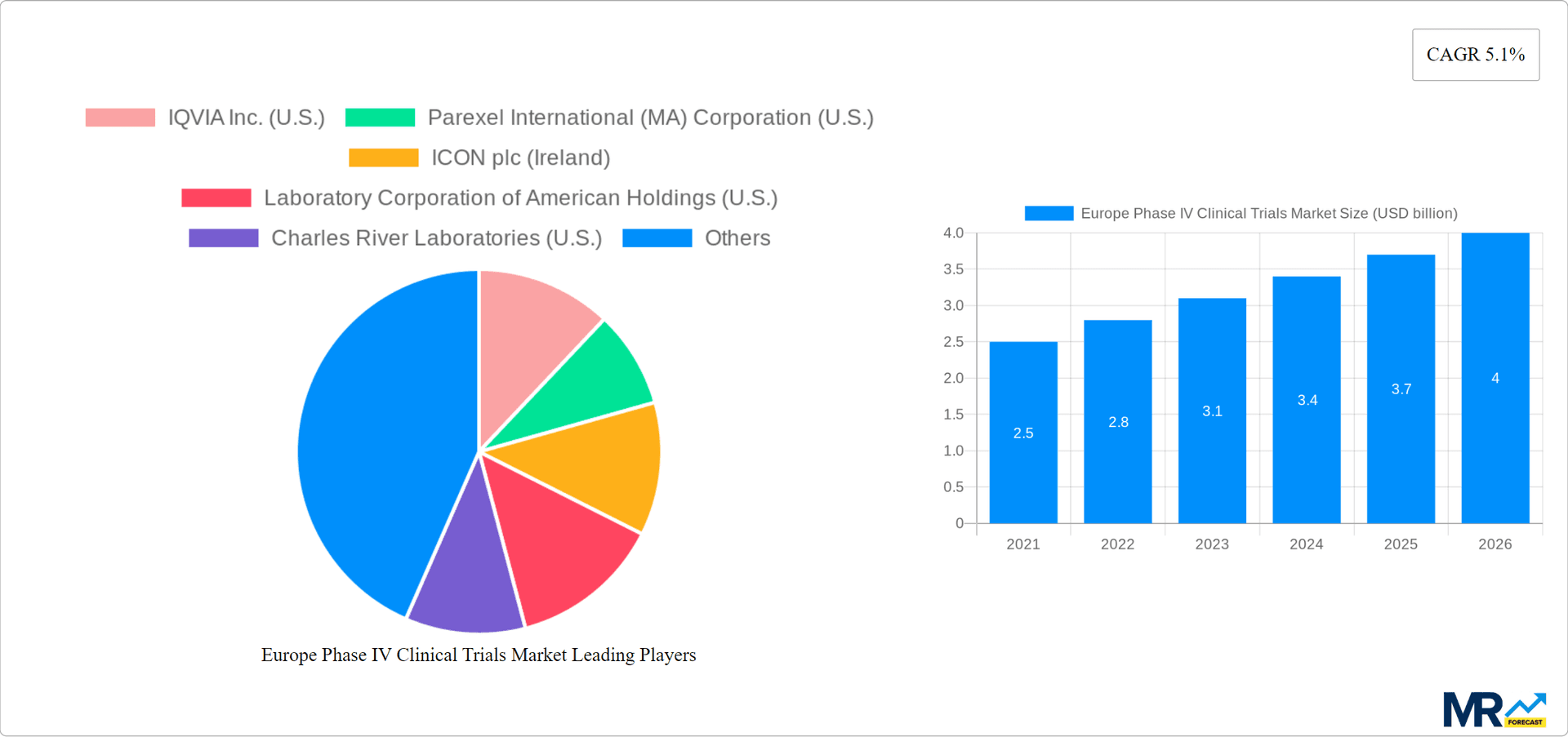
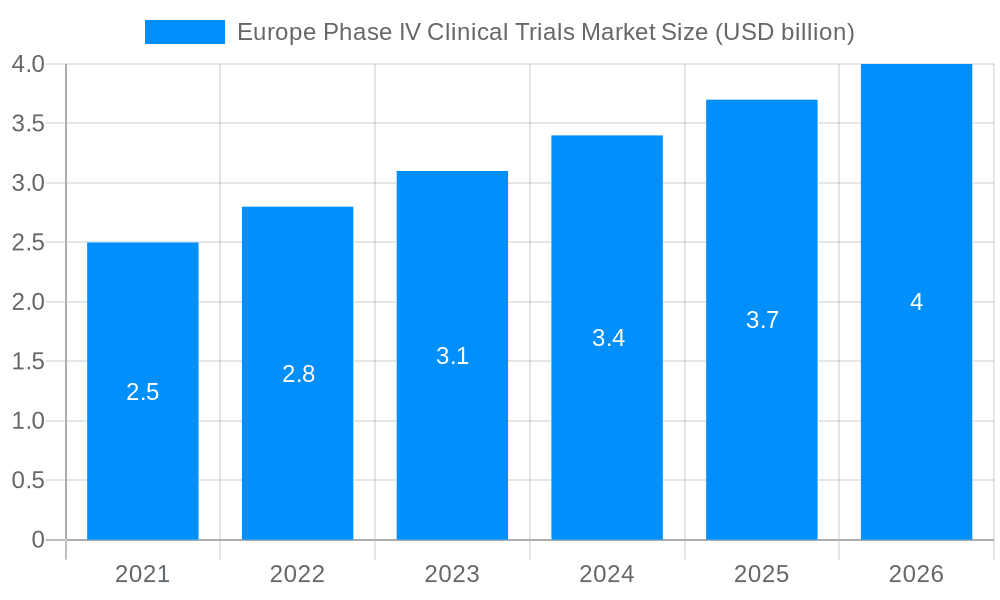
The Europe Phase IV Clinical Trials Market size was valued at USD 2.58 USD billion in 2023 and is projected to reach USD 3.65 USD billion by 2032, exhibiting a CAGR of 5.1 % during the forecast period. The Europe Phase IV Clinical Trials market includes any studies initiated after the approval of a drug or treatment for patient use. The trials are aimed at determining the long-term safety, efficacy, and optimal usage in the healthcare provider's usual facility. They are of utmost importance for assessing therapeutic effectiveness regarding different patient groups and determining the occurrence of rare AE. Their main applications involve drug safety monitoring, providing evidence for uncharted treatment, and comparison treatment choices. Market trends shift to patient-centered results, applying real-world evidence, and adopting digital health technologies for data gathering. A major aim for phase IV trials is safety and effectiveness, alongside growing importance with greater focus of the regulatory bodies on post-marketing surveillance.


Deployment:
Disease Indication:
Type:
This in-depth report offers a thorough examination of the Europe Phase IV Clinical Trials Market, providing actionable insights for stakeholders. The analysis encompasses:
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 5.1% from 2020-2034 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately 5.1%.
Key companies in the market include IQVIA Inc. (U.S.), Parexel International (MA) Corporation (U.S.), ICON plc (Ireland), Laboratory Corporation of American Holdings (U.S.), Charles River Laboratories (U.S.), PHASTAR (U.K.), PSI (Switzerland), Medpace (U.S.), Worldwide Clinical Trials (U.S.), ProRelix Services LLP (U.S.), Sofpromed (Spain).
The market segments include Deployment, Disease Indication, Type.
The market size is estimated to be USD 2.58 USD billion as of 2022.
Rising Prevalence of ASD to Propel Market Growth.
Increasing Number of Hospitals and ASCs Identified as Significant Market Trend.
Challenges Faced During Clinical Trials Hampers Market Growth.
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3850, USD 4850, and USD 5850 respectively.
The market size is provided in terms of value, measured in USD billion.
Yes, the market keyword associated with the report is "Europe Phase IV Clinical Trials Market," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Europe Phase IV Clinical Trials Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.