1. What is the projected Compound Annual Growth Rate (CAGR) of the Electronic Tourniquet?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Electronic Tourniquet
Electronic TourniquetElectronic Tourniquet by Type (Single Channel, Dual Channel, World Electronic Tourniquet Production ), by Application (Hospital, Clinic, Other), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
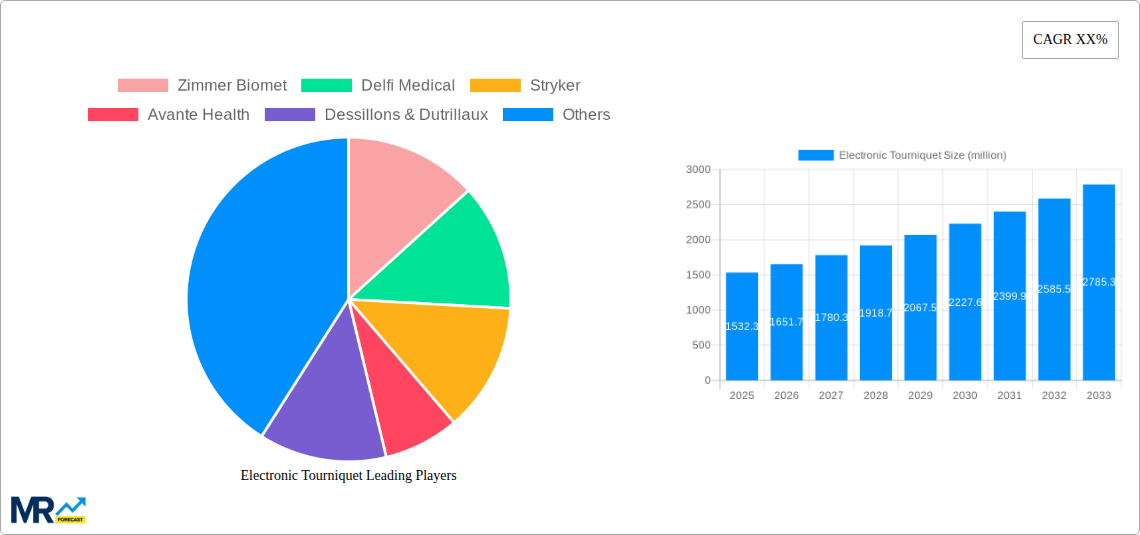
The global Electronic Tourniquet market is poised for substantial growth, projected to reach a market size of approximately $1532.3 million by 2025. This expansion is driven by a confluence of factors, including the increasing prevalence of surgical procedures worldwide, a growing demand for advanced medical devices that enhance patient safety and surgical efficiency, and the continuous technological advancements in medical equipment manufacturing. The market is experiencing a Compound Annual Growth Rate (CAGR) of roughly 7.8%, indicating a robust and sustained upward trajectory. This growth is fueled by the adoption of electronic tourniquets over traditional pneumatic models due to their superior precision, user-friendliness, and ability to provide real-time data monitoring, which are crucial for optimizing surgical outcomes and minimizing complications. The rising healthcare expenditure across both developed and developing economies further bolsters the market, as healthcare facilities are increasingly investing in state-of-the-art technology to improve patient care and operational effectiveness.
The market is segmented into Type and Application, with Dual Channel electronic tourniquets expected to witness higher adoption owing to their advanced capabilities for simultaneous limb occlusion and improved safety profiles. In terms of application, Hospitals are anticipated to dominate the market share, reflecting the high volume of surgical interventions performed within these institutions. Clinics also represent a significant segment, particularly for outpatient procedures. Geographically, North America and Europe are currently leading the market, driven by well-established healthcare infrastructures, high adoption rates of advanced medical technologies, and favorable reimbursement policies. However, the Asia Pacific region is expected to emerge as the fastest-growing market due to rapid advancements in healthcare facilities, increasing medical tourism, and a growing awareness of minimally invasive surgical techniques. Key players like Zimmer Biomet, Stryker, and Delfi Medical are actively investing in research and development to introduce innovative products and expand their global footprint, further stimulating market growth and competition.
This comprehensive report delves into the dynamic global Electronic Tourniquet market, offering an in-depth analysis of its historical performance, current standing, and projected trajectory. Spanning the Study Period of 2019-2033, with a specific focus on the Base Year of 2025, this research provides crucial insights for stakeholders navigating this evolving sector. The Estimated Year of 2025 highlights the current market landscape, while the Forecast Period of 2025-2033 details future growth prospects. Historical trends from 2019-2024 are thoroughly examined to provide a solid foundation for forecasting. The report meticulously analyzes the World Electronic Tourniquet Production, key market drivers, challenges, regional dominance, and critical industry developments.
XXX The Electronic Tourniquet market is experiencing a significant surge, driven by an increasing emphasis on patient safety and surgical precision. The transition from traditional mechanical tourniquets to advanced electronic devices is a defining trend, offering superior control, real-time monitoring, and reduced risk of complications. This shift is fueled by technological advancements in microprocessor control, pressure sensors, and user-friendly interfaces, enhancing their adoption across various surgical specialties. The growing prevalence of minimally invasive surgical procedures, which often necessitate precise and controlled blood occlusion, further amplifies demand for these sophisticated devices. Furthermore, increasing healthcare expenditure globally, coupled with a rising awareness among healthcare professionals about the benefits of electronic tourniquets in improving patient outcomes and reducing post-operative morbidity, is contributing to market expansion. The market is also witnessing a diversification in product offerings, with manufacturers focusing on developing single-channel and dual-channel devices to cater to a broader spectrum of surgical needs, from routine limb surgeries to complex orthopedic procedures. The integration of data logging and connectivity features is another emerging trend, allowing for better documentation, performance analysis, and potential integration into hospital information systems. This trend towards smart medical devices underscores the growing importance of digital health solutions in enhancing surgical efficiency and patient care. The global electronic tourniquet production is projected to witness substantial growth, with the market size expected to reach significant figures in the coming years. The increasing sophistication of these devices, offering enhanced safety features like pre-set pressure limits and automatic deflation alarms, is making them indispensable in modern surgical settings. The growing understanding of tourniquet-induced nerve damage and tissue injury is prompting a greater preference for electronic devices that offer more predictable and controlled cuff inflation and deflation. This meticulous control minimizes the risk of complications, thereby improving the overall surgical experience for both patients and surgeons. The market is also being shaped by the increasing demand from emerging economies, where healthcare infrastructure is rapidly developing, and advanced medical technologies are being readily adopted. The competitive landscape is characterized by a mix of established players and emerging manufacturers, all vying to capture market share through product innovation, strategic partnerships, and robust distribution networks. The increasing focus on patient recovery and rehabilitation post-surgery is also indirectly driving the demand for electronic tourniquets, as their precise application can contribute to better surgical outcomes and faster recovery times. The market's growth trajectory is further bolstered by regulatory bodies' increasing emphasis on patient safety standards, encouraging the adoption of evidence-based medical devices like electronic tourniquets. The rising incidence of orthopedic surgeries, a major application area for tourniquets, is a direct contributor to the market's expansion. The global electronic tourniquet market is anticipated to grow steadily, fueled by continuous innovation and increasing adoption rates in healthcare facilities worldwide.
The electronic tourniquet market is experiencing robust growth driven by several key factors. Foremost among these is the paramount importance placed on patient safety and the reduction of surgical complications. Electronic tourniquets offer superior control over occlusion pressure and duration compared to their manual counterparts, significantly minimizing the risk of nerve damage, tissue injury, and other adverse events. This enhanced safety profile makes them the preferred choice in an era of increasing medical malpractice awareness and stringent healthcare regulations. Secondly, the escalating volume of surgical procedures, particularly orthopedic surgeries, trauma care, and reconstructive procedures, directly translates to a higher demand for reliable and effective occlusion devices. The aging global population, with its associated increase in degenerative joint diseases and trauma-related injuries, further fuels this demand. Technological advancements are also a significant propellant. The integration of sophisticated microprocessors, accurate pressure sensors, and intuitive user interfaces has transformed electronic tourniquets into sophisticated medical instruments. These advancements enable precise pressure management, real-time monitoring, and customizable settings, catering to the specific needs of diverse surgical interventions. Furthermore, the growing trend towards minimally invasive surgery (MIS) indirectly benefits the electronic tourniquet market. MIS techniques often require meticulous control and visualization, and the reliable occlusion provided by electronic tourniquets is crucial for optimal surgical outcomes in these procedures. The increasing adoption of advanced healthcare technologies and the growing focus on evidence-based medicine within healthcare institutions globally are also contributing to the upward trajectory of this market.

Despite the promising growth trajectory, the electronic tourniquet market faces several challenges and restraints that could impede its full potential. A primary concern is the high initial cost of electronic tourniquets. Compared to traditional mechanical tourniquets, electronic devices are significantly more expensive, which can be a substantial barrier for smaller clinics, hospitals in resource-limited settings, and developing economies. This cost factor can lead to a slower adoption rate, particularly in regions where budget constraints are a significant consideration. Another challenge lies in the need for specialized training and technical expertise. While user interfaces are becoming more intuitive, operating and maintaining electronic tourniquets effectively requires trained personnel to ensure proper functionality and prevent misuse, which could lead to complications. The risk of device malfunction or failure also presents a restraint. Although rare, any electronic device is susceptible to technical glitches, power outages, or battery failures. Such failures during a critical surgical procedure can have severe consequences, necessitating robust backup systems and stringent quality control measures from manufacturers. Furthermore, the availability of cheaper and simpler mechanical alternatives continues to pose a competitive threat, especially for less complex surgical procedures where the added benefits of electronic control may not be perceived as essential by some practitioners. Regulatory hurdles and the lengthy approval processes for new medical devices in various countries can also slow down market entry and expansion for manufacturers. Finally, while increasing, awareness and acceptance of electronic tourniquets may not be uniform across all geographical regions and among all surgical specialties. Some surgeons might remain accustomed to traditional methods, requiring concerted efforts in education and demonstration of the technology's advantages.
The global Electronic Tourniquet market is poised for significant growth, with several key regions and segments expected to lead the charge. Among the Segments, the Hospital application segment is projected to hold a dominant share of the market.
Hospitals: These institutions, particularly larger tertiary care centers and teaching hospitals, are characterized by a high volume of surgical procedures, advanced infrastructure, and the financial capacity to invest in cutting-edge medical technology. The increasing focus on patient safety protocols, the availability of specialized surgical teams performing complex procedures, and the continuous drive for improved surgical outcomes make hospitals the primary adopters of electronic tourniquets. The prevalence of orthopedic, trauma, and vascular surgeries, all of which significantly benefit from precise tourniquet control, is particularly high in hospital settings. Furthermore, hospitals are often at the forefront of adopting new medical technologies due to their research-oriented nature and the presence of influential opinion leaders who can champion new devices. The demand for both single-channel and dual-channel electronic tourniquets is substantial within hospitals, catering to a wide range of surgical needs. The investment in these devices is often driven by the long-term benefits of reduced complication rates, shorter patient recovery times, and improved overall efficiency in the operating room.
World Electronic Tourniquet Production: Countries with a strong medical device manufacturing base and a significant presence of R&D facilities are expected to dominate the production landscape. This includes regions with established expertise in producing high-precision medical instruments.
Dual Channel Segment: While single-channel devices will continue to see demand, the dual-channel segment is anticipated to experience a higher growth rate. This is due to the increased versatility and enhanced safety features offered by dual-channel systems, which allow for independent limb occlusion and deflation, proving invaluable in complex or bilateral limb surgeries.
Geographically, North America is expected to remain a dominant region in the electronic tourniquet market.
Other regions, such as Europe, are also significant contributors to the market.
The Asia Pacific region, while currently smaller, is projected to witness the fastest growth rate.
The dominance of these regions and segments is underpinned by a confluence of factors including technological advancements, economic capabilities, healthcare demands, and a continuous pursuit of improved patient care standards.
Several key factors are acting as potent growth catalysts for the electronic tourniquet industry. The accelerating global demand for orthopedic, trauma, and reconstructive surgeries, driven by an aging population and rising incidences of related conditions, is a primary driver. Furthermore, a heightened awareness among healthcare professionals regarding the significant benefits of electronic tourniquets in minimizing surgical complications, such as nerve damage and tissue injury, is encouraging their adoption. The continuous innovation and development of more sophisticated, user-friendly, and cost-effective electronic tourniquet systems by manufacturers are also crucial catalysts, making these devices more accessible and appealing. The increasing investment in healthcare infrastructure, particularly in emerging economies, is opening up new markets and opportunities for growth.
The electronic tourniquet market is populated by a number of key companies that are driving innovation and market growth. Some of the prominent players include:
The electronic tourniquet sector has witnessed several significant developments that have shaped its evolution:
This report provides a comprehensive and detailed analysis of the Electronic Tourniquet market, offering invaluable insights for stakeholders. It meticulously examines market dynamics, including drivers, restraints, and opportunities, and provides granular data on market segmentation by type, application, and region. With a robust study period encompassing 2019-2033, including the Base Year of 2025 and a detailed Forecast Period of 2025-2033, the report delivers forward-looking projections grounded in historical analysis from 2019-2024. It identifies key industry trends, analyzes the competitive landscape with a focus on leading players, and highlights significant technological advancements and developments. This report serves as an indispensable resource for manufacturers, distributors, healthcare providers, investors, and policymakers seeking to understand and capitalize on the opportunities within the global electronic tourniquet market.

| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Zimmer Biomet, Delfi Medical, Stryker, Avante Health, Dessillons & Dutrillaux, AneticAid, VBM, Advin Health Care, Staan Bio-Med Engineering, Weihai Bohua Medical, Jinan Fule Medical Instrument, Changzhou Yanling Electronic Equipment, .
The market segments include Type, Application.
The market size is estimated to be USD 1532.3 million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4480.00, USD 6720.00, and USD 8960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Electronic Tourniquet," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Electronic Tourniquet, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.