1. What is the projected Compound Annual Growth Rate (CAGR) of the Circumcision Suture Device?
The projected CAGR is approximately XX%.
MR Forecast provides premium market intelligence on deep technologies that can cause a high level of disruption in the market within the next few years. When it comes to doing market viability analyses for technologies at very early phases of development, MR Forecast is second to none. What sets us apart is our set of market estimates based on secondary research data, which in turn gets validated through primary research by key companies in the target market and other stakeholders. It only covers technologies pertaining to Healthcare, IT, big data analysis, block chain technology, Artificial Intelligence (AI), Machine Learning (ML), Internet of Things (IoT), Energy & Power, Automobile, Agriculture, Electronics, Chemical & Materials, Machinery & Equipment's, Consumer Goods, and many others at MR Forecast. Market: The market section introduces the industry to readers, including an overview, business dynamics, competitive benchmarking, and firms' profiles. This enables readers to make decisions on market entry, expansion, and exit in certain nations, regions, or worldwide. Application: We give painstaking attention to the study of every product and technology, along with its use case and user categories, under our research solutions. From here on, the process delivers accurate market estimates and forecasts apart from the best and most meaningful insights.
Products generically come under this phrase and may imply any number of goods, components, materials, technology, or any combination thereof. Any business that wants to push an innovative agenda needs data on product definitions, pricing analysis, benchmarking and roadmaps on technology, demand analysis, and patents. Our research papers contain all that and much more in a depth that makes them incredibly actionable. Products broadly encompass a wide range of goods, components, materials, technologies, or any combination thereof. For businesses aiming to advance an innovative agenda, access to comprehensive data on product definitions, pricing analysis, benchmarking, technological roadmaps, demand analysis, and patents is essential. Our research papers provide in-depth insights into these areas and more, equipping organizations with actionable information that can drive strategic decision-making and enhance competitive positioning in the market.
 Circumcision Suture Device
Circumcision Suture DeviceCircumcision Suture Device by Type (Type 12, Type 18, Type 26, Type 30, Type 36), by Application (Hospital, Clinic, Others), by North America (United States, Canada, Mexico), by South America (Brazil, Argentina, Rest of South America), by Europe (United Kingdom, Germany, France, Italy, Spain, Russia, Benelux, Nordics, Rest of Europe), by Middle East & Africa (Turkey, Israel, GCC, North Africa, South Africa, Rest of Middle East & Africa), by Asia Pacific (China, India, Japan, South Korea, ASEAN, Oceania, Rest of Asia Pacific) Forecast 2025-2033
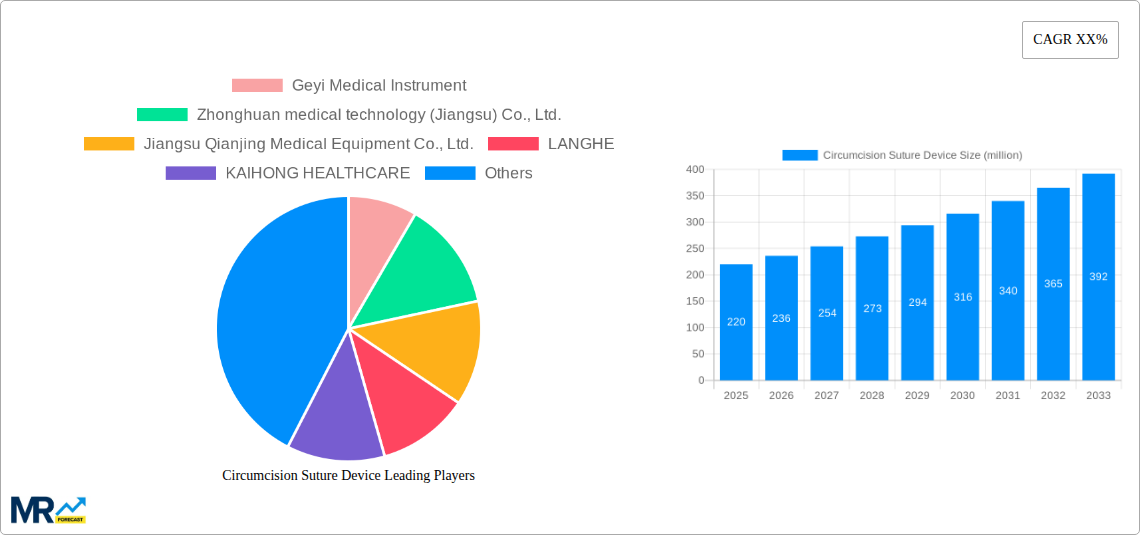
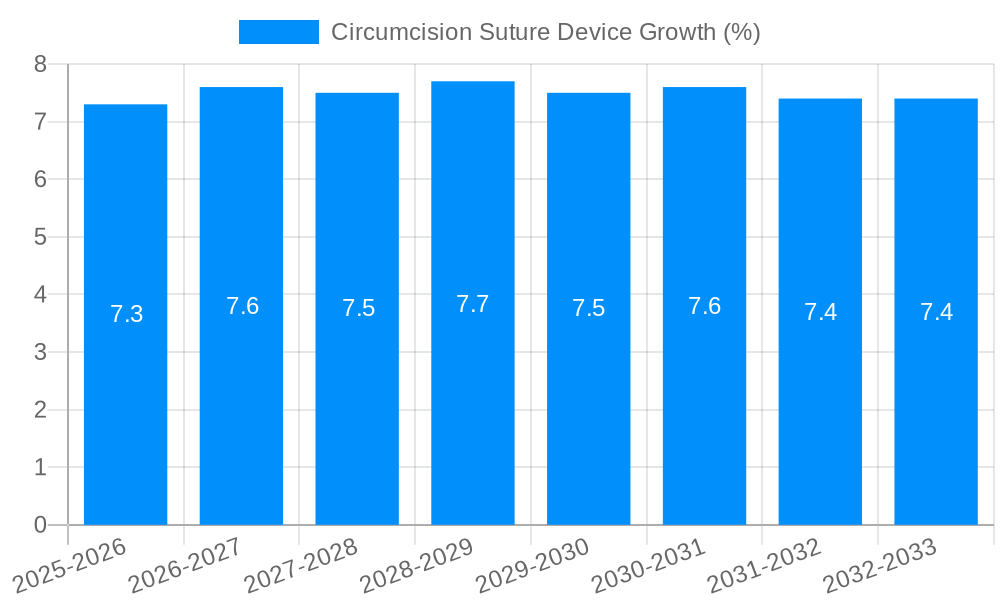
The global Circumcision Suture Device market is poised for significant growth, projected to reach approximately $350 million by 2033, exhibiting a Compound Annual Growth Rate (CAGR) of around 7.5% from its estimated 2025 valuation. This expansion is largely driven by the increasing awareness of male circumcision for health and hygiene purposes, particularly in regions with high birth rates and established cultural practices. The rising incidence of foreskin-related conditions and the subsequent demand for minimally invasive and efficient surgical solutions are key accelerators for this market. Furthermore, advancements in device technology, leading to enhanced safety, reduced procedure time, and improved patient outcomes, are also contributing to market buoyancy. The market's progression is also influenced by the increasing adoption of these devices in both hospital settings and outpatient clinics, catering to a wider patient demographic and diverse healthcare environments.
The market's growth trajectory faces certain restraints, including stringent regulatory approvals and the high cost associated with some advanced devices, which can limit their widespread adoption, especially in price-sensitive economies. However, the anticipated growth in emerging markets, coupled with the continuous innovation pipeline from key players like Geyi Medical Instrument, Zhonghuan medical technology, and Henry Schein, is expected to mitigate these challenges. The market segmentation reveals a strong preference for Type 26 and Type 30 devices, likely due to their perceived efficacy and ease of use. Geographically, the Asia Pacific region, led by China and India, is expected to dominate the market share owing to its large population and growing healthcare expenditure. North America and Europe also represent substantial markets, driven by established healthcare infrastructures and a focus on advanced medical technologies.
Here is a unique report description on Circumcision Suture Devices, incorporating your specified elements:
This report offers an in-depth exploration of the global Circumcision Suture Device market, providing critical insights and forecasts for stakeholders. The study meticulously analyzes trends, driving forces, challenges, regional dominance, and key players from the historical period of 2019-2024 through to a projected horizon extending to 2033, with a base and estimated year of 2025. With an estimated market size in the hundreds of millions of units, this report delves into the nuanced dynamics shaping the future of this specialized medical device sector.
The Circumcision Suture Device market is experiencing a dynamic evolution, characterized by a discernible shift towards innovative, minimally invasive solutions. Historically, traditional suturing techniques have been prevalent, but advancements in device design are now facilitating quicker, more precise, and less traumatic circumcisions. This trend is particularly evident in the increasing adoption of advanced closure devices that streamline the procedure, reduce operative time, and minimize the risk of complications such as bleeding and infection. The demand is being shaped by a growing awareness of improved post-operative outcomes, leading healthcare providers and parents to seek devices that offer superior aesthetic results and faster patient recovery.
Furthermore, the market is witnessing a segmentation driven by different device types. While historically less specific, the emergence of distinct Type 12, Type 18, Type 26, Type 30, and Type 36 devices caters to a wider range of patient anatomies and procedural preferences. This specialization allows for a more tailored approach, enhancing efficacy and patient comfort. The growing preference for these specialized types underscores the maturing nature of the market and the increasing sophistication of surgical practices. The market's trajectory is also influenced by varying regulatory landscapes and healthcare policies across different geographies, impacting the adoption rates of specific technologies. Global health initiatives promoting male circumcision for medical and cultural reasons continue to underpin the consistent demand for these devices. The report anticipates a sustained growth pattern driven by these underlying trends, with an increasing focus on cost-effectiveness without compromising on quality and safety.
Several key factors are propelling the growth of the Circumcision Suture Device market. Foremost among these is the increasing global prevalence of male circumcision, driven by a confluence of medical, religious, and cultural factors. Medically, the established benefits of male circumcision in reducing the risk of certain infections, including HIV, urinary tract infections, and penile cancer, continue to be a significant driver, particularly in regions with higher prevalence rates of these conditions. Furthermore, religious mandates and cultural traditions across various communities worldwide ensure a consistent and substantial demand for circumcision procedures.
The technological advancements in the design and functionality of circumcision suture devices are also a major impetus. Manufacturers are continuously innovating to develop devices that are safer, more efficient, and less invasive than traditional methods. This includes the introduction of devices that offer improved precision in wound closure, reduced scarring, and faster healing times. The focus on patient comfort and minimizing post-operative pain and recovery periods is a critical element in driving the adoption of these advanced devices. The growing awareness among healthcare providers and patients about these benefits, coupled with the increasing accessibility of these devices in both Hospital and Clinic settings, further strengthens the market's upward trajectory. The expanding healthcare infrastructure in developing nations, coupled with rising disposable incomes, also contributes to greater access to these procedures and devices.
Despite the robust growth drivers, the Circumcision Suture Device market is not without its challenges and restraints. A significant hurdle is the prevailing cost of advanced circumcision suture devices. While offering superior benefits, their higher price point compared to traditional suturing materials can be a deterrent, especially in price-sensitive markets or for healthcare facilities with limited budgets. This cost factor can lead to a slower adoption rate of the latest technologies in certain regions.
Another considerable restraint is the cultural and religious opposition to male circumcision in some parts of the world. While medical and cultural practices drive demand elsewhere, ethical debates and anti-circumcision movements in certain countries can significantly limit the market size and growth potential in those specific geographies. Furthermore, the availability of skilled healthcare professionals trained in the use of specific advanced circumcision suture devices can be a bottleneck. Improper usage or lack of expertise can lead to complications, thereby impacting the perceived safety and efficacy of these devices and hindering wider acceptance. Stringent regulatory approvals and the time-consuming processes associated with obtaining these can also act as a restraint, delaying the market entry of new and innovative products. The report identifies these factors as crucial considerations for stakeholders navigating the complexities of this market.
The global Circumcision Suture Device market is poised for significant dominance by specific regions and product segments, driven by a complex interplay of medical needs, cultural practices, and economic development.
Dominant Segments:
Type 36 Devices: This specific type of circumcision suture device is anticipated to witness substantial market share. The Type 36 devices often represent a more advanced, potentially larger-capacity, or more versatile design compared to smaller types. This versatility makes them suitable for a broader range of patient anatomies and procedural scenarios, leading to higher adoption rates in diverse clinical settings. Their design likely incorporates enhanced features for precision and ease of use, addressing the evolving demands for less invasive and more efficient surgical outcomes. This type is particularly favored in established healthcare systems where technological advancement is prioritized.
Hospital Application: The Hospital segment is projected to be the largest consumer of circumcision suture devices. Hospitals, being primary healthcare providers, perform a significant volume of these procedures, encompassing both elective circumcisions and those performed for medical indications or as part of neonatal care. The presence of specialized surgical teams, advanced infrastructure, and the capacity to handle complex cases within hospital settings naturally positions them as the leading application area. Furthermore, hospitals often have the purchasing power and are early adopters of newer, more sophisticated medical technologies, including advanced suture devices.
Dominant Region/Country:
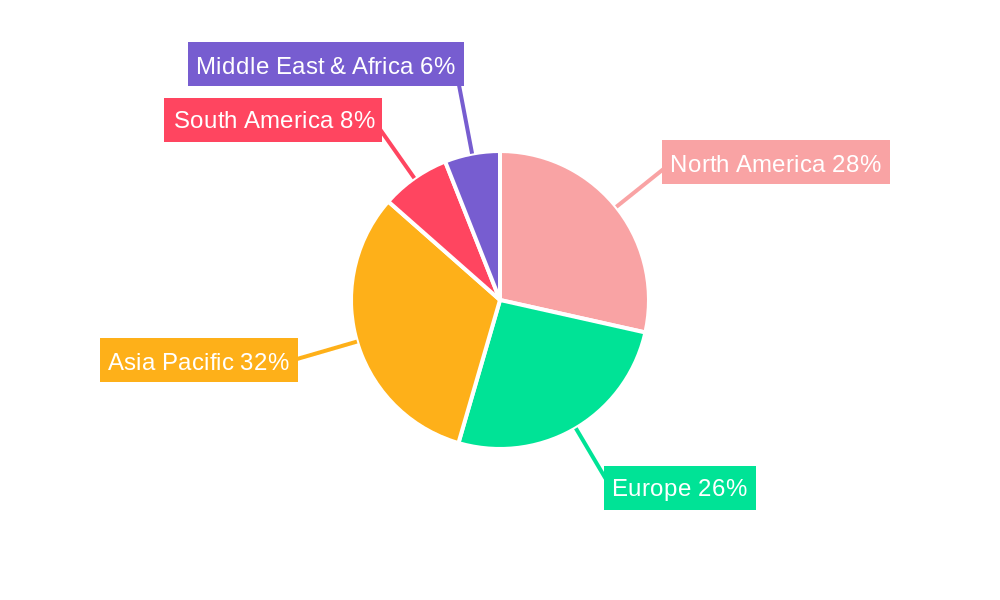
North America: This region is expected to continue its market leadership. The strong emphasis on public health initiatives, high per capita healthcare spending, and a well-established healthcare infrastructure contribute to the sustained demand for advanced medical devices. The acceptance of male circumcision for medical and, in some communities, religious reasons, alongside a proactive approach to preventive healthcare, underpins this dominance. The presence of leading medical device manufacturers and a high rate of adoption of innovative surgical techniques further solidify North America's position. The demand for precise, minimally invasive devices that ensure rapid recovery and minimal scarring is particularly high in this region. The robust reimbursement policies for medical procedures also play a crucial role in enabling access to these devices.
Asia Pacific: While North America leads, the Asia Pacific region is expected to exhibit the fastest growth. This surge is attributed to the large and growing populations, increasing awareness of the medical benefits of circumcision, and the significant influence of cultural and religious practices that mandate the procedure in many countries. Furthermore, the expanding healthcare infrastructure, coupled with rising disposable incomes, is making these devices more accessible. Countries like China and India, with their vast populations and improving healthcare systems, are becoming increasingly important markets. The report details how government initiatives aimed at improving healthcare access and the growing number of private healthcare facilities are contributing to this accelerated growth. The increasing preference for technologically advanced devices for better patient outcomes is also a key factor driving market penetration in this region.
The Circumcision Suture Device industry is experiencing growth catalysts primarily driven by the increasing global acceptance of male circumcision for its preventative health benefits, such as reduced risks of HIV and certain penile cancers. This, coupled with consistent cultural and religious practices mandating the procedure in numerous regions, ensures a steady and growing demand. Technological advancements leading to more efficient, less invasive, and safer devices that reduce operative time and improve patient recovery are also significant catalysts.
This comprehensive report delves into the global Circumcision Suture Device market, offering a granular analysis of its trajectory from 2019 to 2033. It encompasses key market insights, detailed examination of driving forces, critical challenges, and identifies dominant regions and segments, including the anticipated lead of Type 36 devices and Hospital applications. The report further highlights growth catalysts, lists leading market players, and details significant historical and projected developments, providing a holistic understanding of market dynamics and future opportunities. With an estimated market size in the hundreds of millions of units, this report is an indispensable resource for stakeholders seeking to navigate this evolving sector.
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|




Note*: In applicable scenarios
Primary Research
Secondary Research

Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence
The projected CAGR is approximately XX%.
Key companies in the market include Geyi Medical Instrument, Zhonghuan medical technology (Jiangsu) Co., Ltd., Jiangsu Qianjing Medical Equipment Co., Ltd., LANGHE, KAIHONG HEALTHCARE, Nanchang YiLi Medical Instrument Co.,LTD, Tonglu Kanger Medical Instrument Co.,Ltd., Henry Schein, Precision Medical, Changzhou 3R Medical Device Technology Co., Ltd, Fruugo, Accel Medical, Medzell, CONTINENTAL SUPPLIES LTD., Sinolinks Medical Innovatoin, Inc., Flipkart, Jiangsu Sunride Medical Technology Co., Ltd..
The market segments include Type, Application.
The market size is estimated to be USD XXX million as of 2022.
N/A
N/A
N/A
N/A
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3480.00, USD 5220.00, and USD 6960.00 respectively.
The market size is provided in terms of value, measured in million and volume, measured in K.
Yes, the market keyword associated with the report is "Circumcision Suture Device," which aids in identifying and referencing the specific market segment covered.
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
To stay informed about further developments, trends, and reports in the Circumcision Suture Device, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.